9) Which of the following reactions will not occur as written?
A) Zn (s) + Pb(NO3)2 (aq) → Pb (s) + Zn(NO3)2 (aq)
B) Mg (s) + Ca(OH)2 (aq) → Ca (s) + Mg(OH)2 (aq)
C) Sn (s) + 2AgNO3 (aq) → 2Ag (s) + Sn(NO3)2 (aq)
D) Co (s) + 2AgCl (aq) → 2Ag (s) + CoCl2 (aq)
E) Co (s) + 2HI (aq) → H2 (g) + CoI2 (aq)
10) The bond angles marked a, b, and c in the molecule below are about ________,
________, and ________, respectively.
A) 109.5°, 109.5°, 109.5°
B) 120°, 109.5°, 120°
C) 109.5°, 109.5°, 120°
D) 90°, 180°, 90°
E) 109.5°, 109.5°, 90°
11) The ________ subshell contains only one orbital.
A) 5d
B) 6f
C) 4s
D) 3d
E) 1p
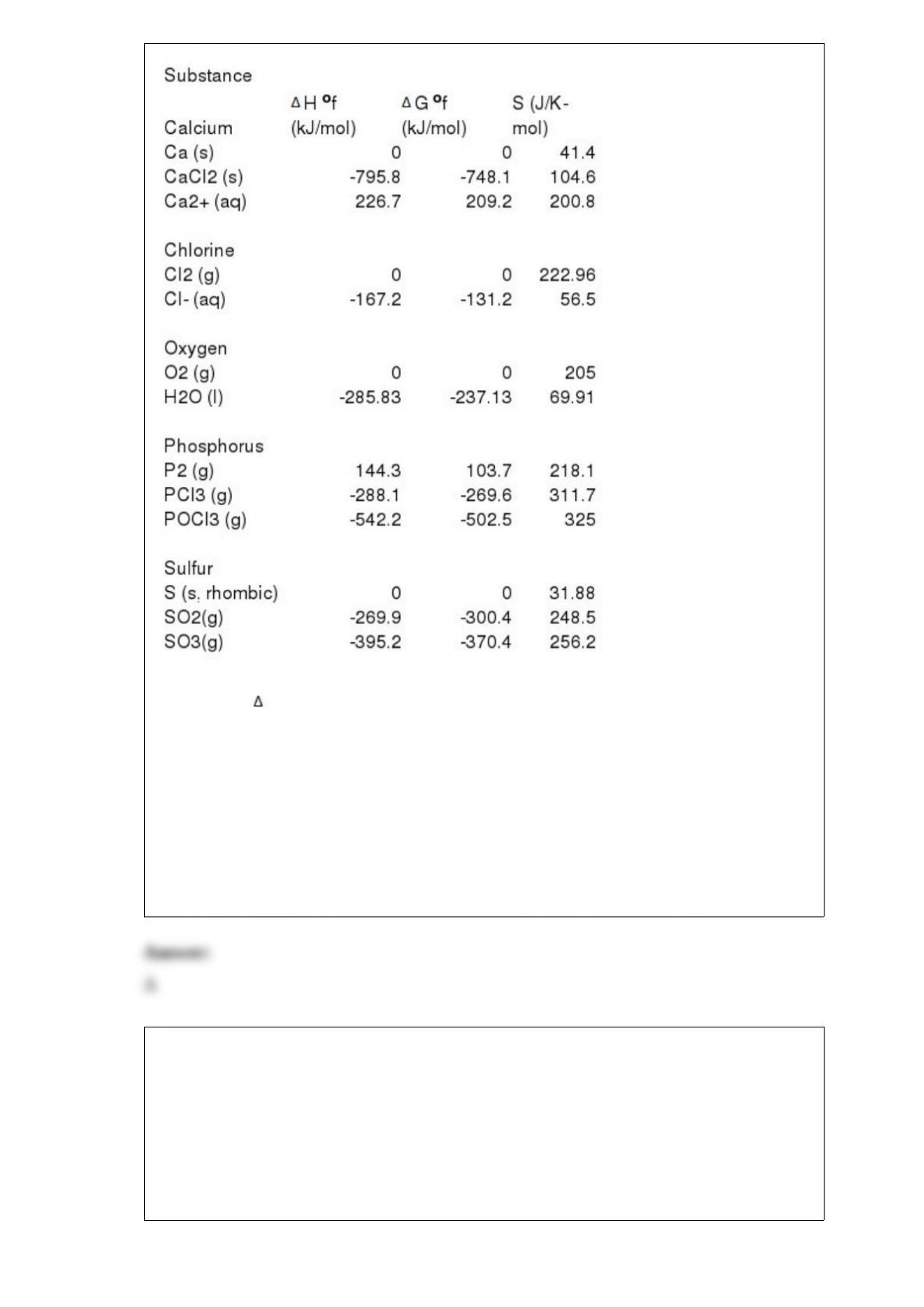
12) Thermodynamic Quantities for Selected Substances at 298.15 K (25 oC)