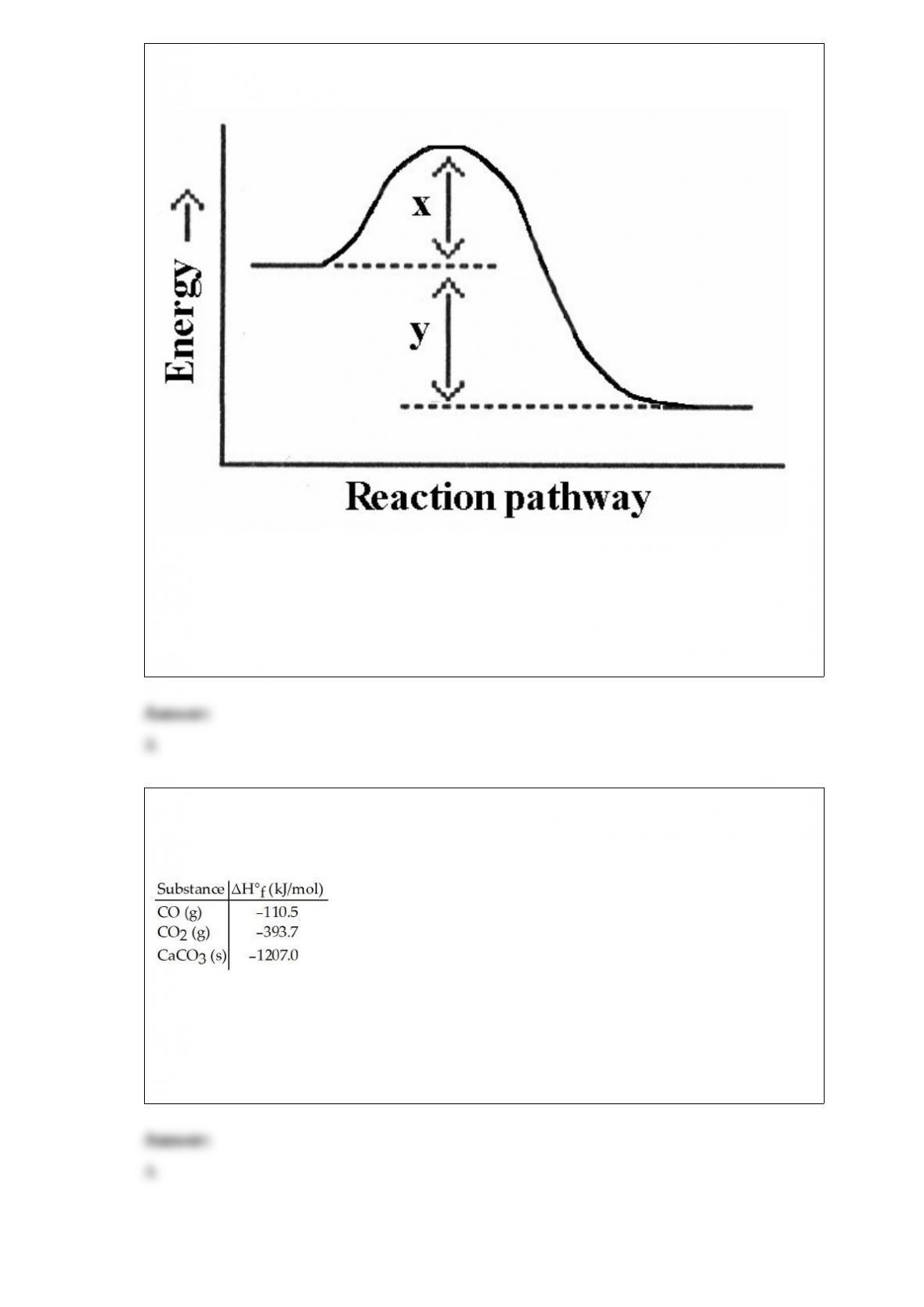
25) Calculate the bond energy of C€F given that the heat of atomization of CHFClBr is
1502 kJ/mol, and that the bond energies of C€H, C€Br, and C€Cl are 413, 276, and 328
kJ/mol, respectively.
26) The world's largest desalinization plant is in ________ and uses the process of
________ to produce drinking water.
27) The relationship of absorbed light to the concentration of the substance absorbing
the light is governed by ________.
28) Water can be formed from the stoichiometric reaction of hydrogen with oxygen:
2H2 (g) + O2 (g) → 2H2O (g)
A complete reaction of 5.0 g of O2 with excess hydrogen produces ________ g of H2O.
29) What is the oxidation state of the iron atom in CaNa[Fe(CN)6]?