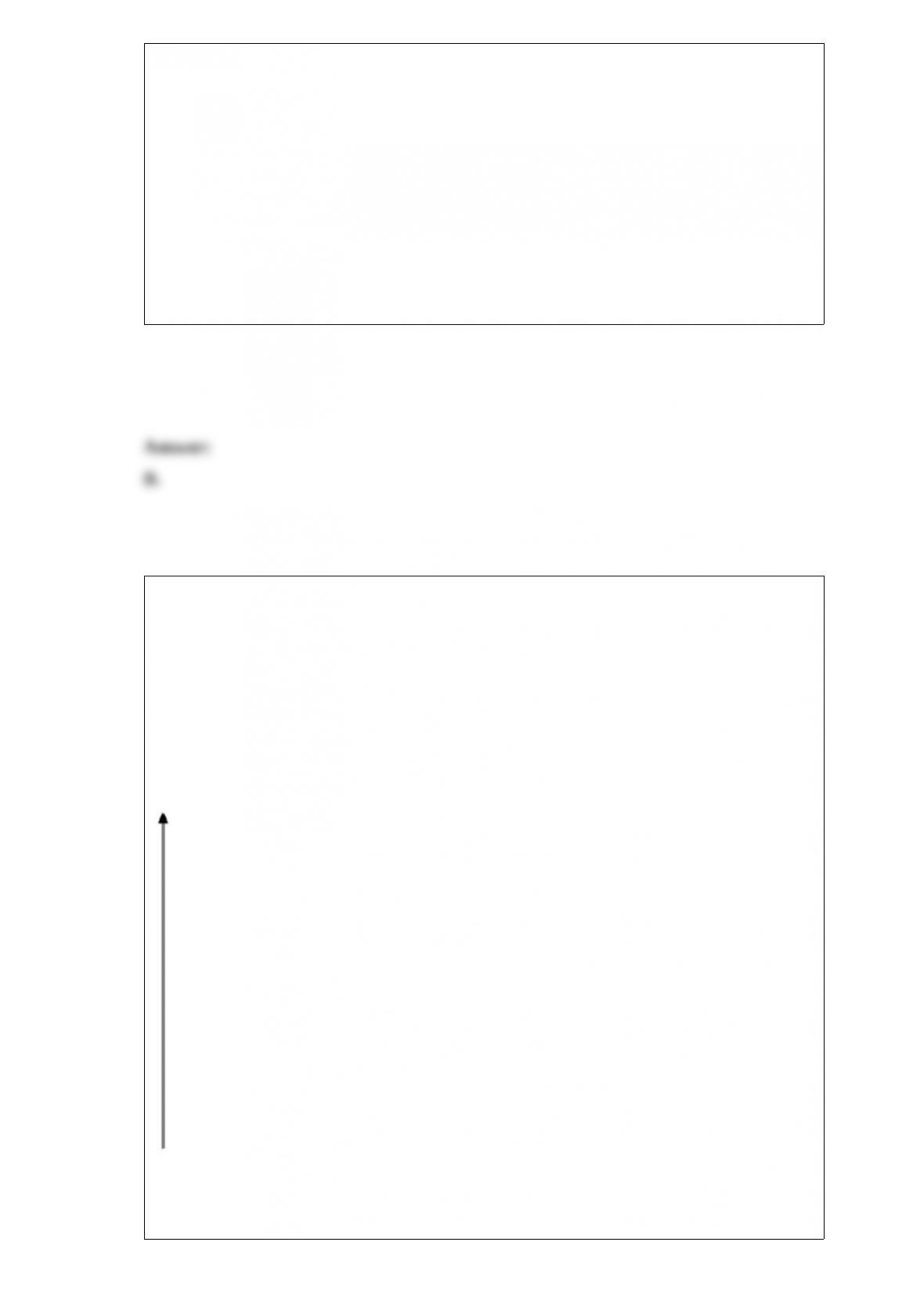
5.2895 g 5.0001
g
5.1077 g 4.9998
g
4.6407 g 4.9999
g
4.8947 g 5.0002
g
5.0672 g 4.9999
g
average mass = 5.0000 g 5.0000
g
Which statement best describes the results?
A. A: poor precision, good accuracy. B: good precision, good accuracy.
B. A: good precision, good accuracy. B: poor precision, good accuracy.
C. A: good precision, good accuracy. B: good precision, poor accuracy.
D. A: poor precision, good accuracy. B: good precision, poor accuracy.
E. A: good precision, poor accuracy. B: poor precision, good accuracy.
If a stress is applied to an equilibrium system, the system will respond in such a way as
to relieve that stress. This is a statement of ________ principle.