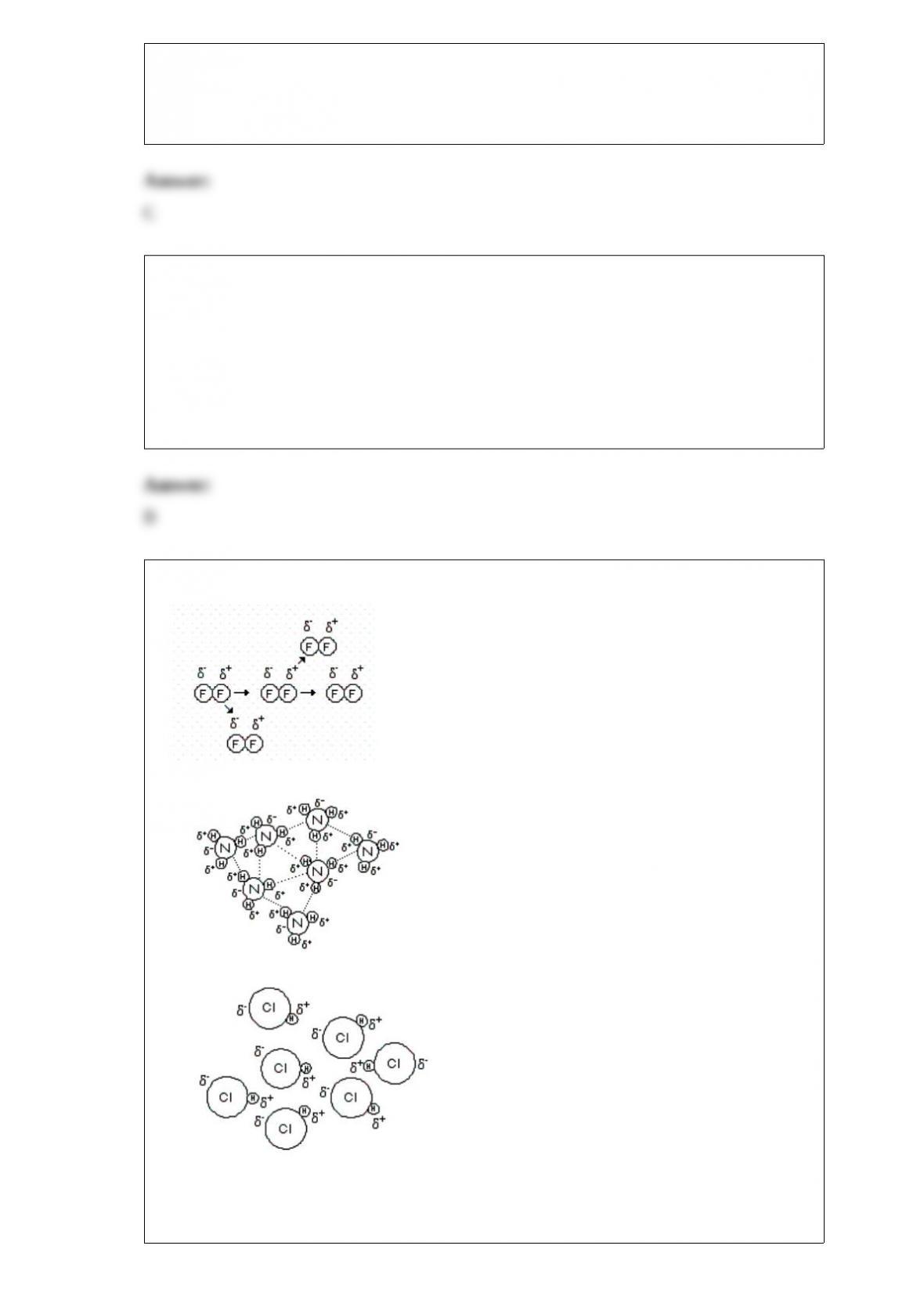
Larger atomic radius, P or Sb
Calculate the osmotic pressure (in torr) of 6.00 L of an aqueous 0.958 M solution at
30.0C, if the solute concerned is totally ionized into three ions (e.g., it could be
Na2SO4or MgCl2).
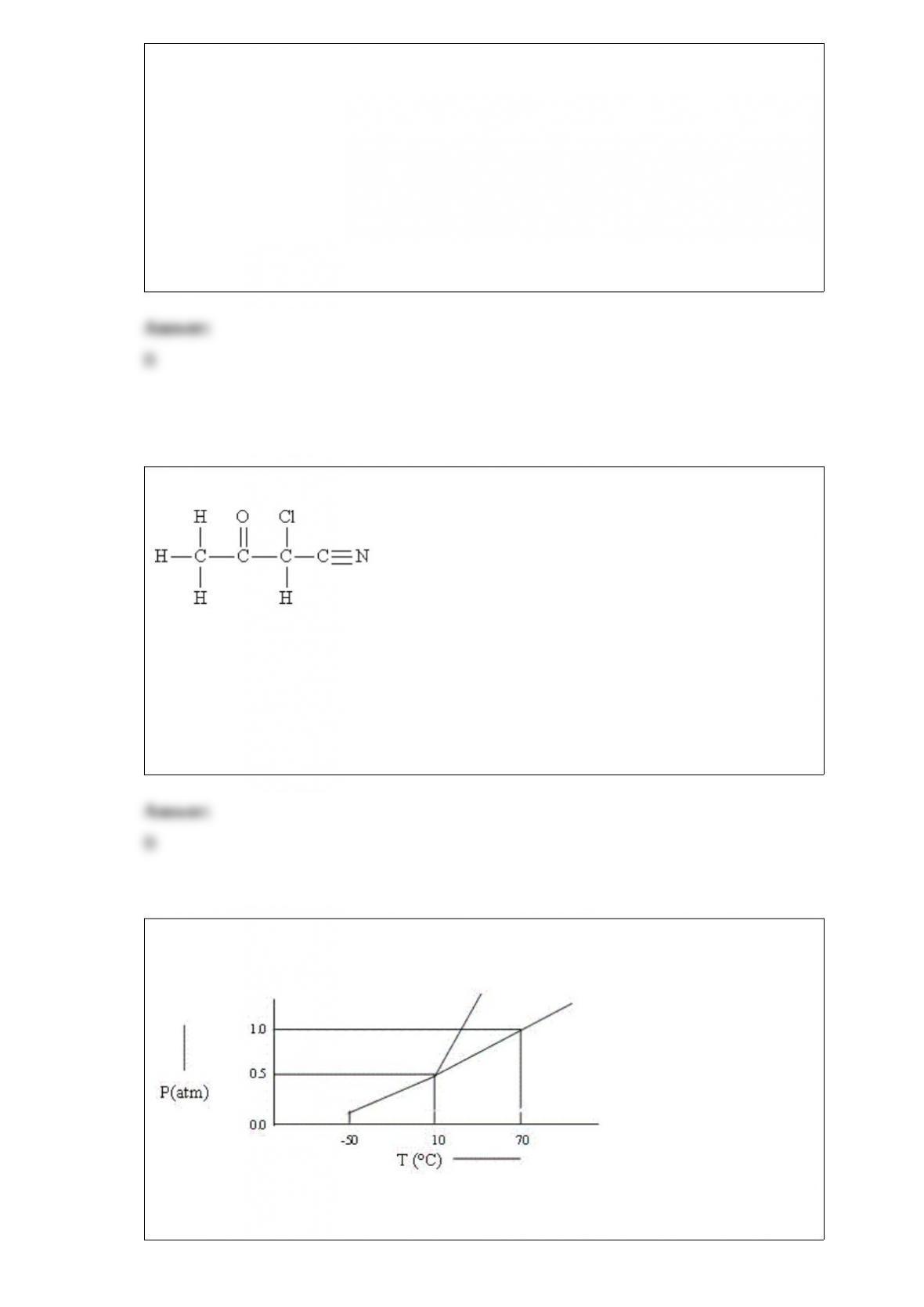
For each of the following compounds:
a)Draw the Lewis structure.
b)Give the shape of the molecule.
c)Indicate the polarity of the molecule.
AlF3
The calcium atom is much larger than the calcium ion, while the fluorine atom is much
smaller than the fluorine ion. Explain this natural occurrence.
A cation has a larger proton to electron ratio than the corresponding neutral atom, so the
remaining electrons are more closely held. An anion has a smaller proton to electron
ratio than its corresponding neutral atom, so the electrons can not be held as closely.
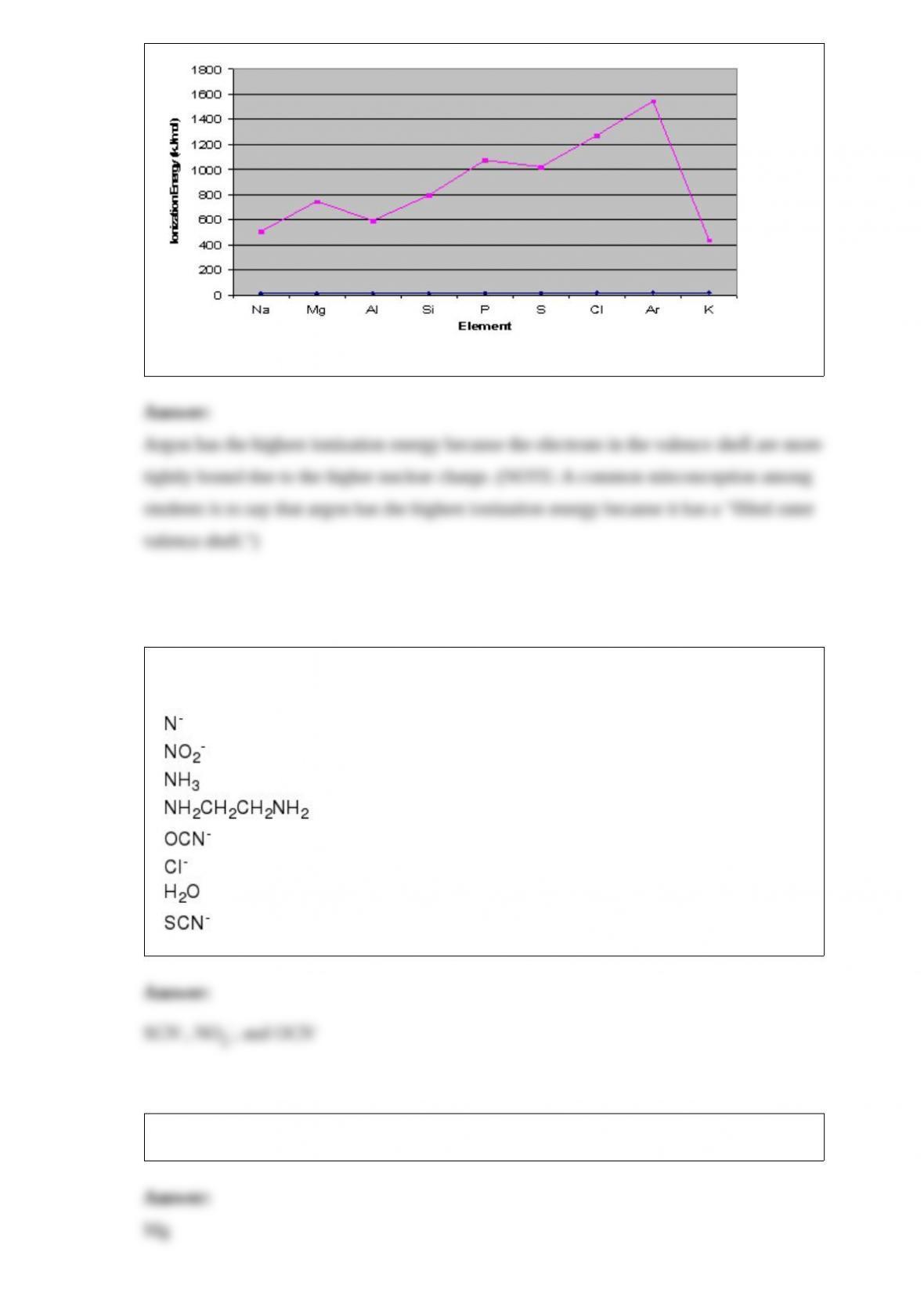
Consider the graph below to answer the next two questions: