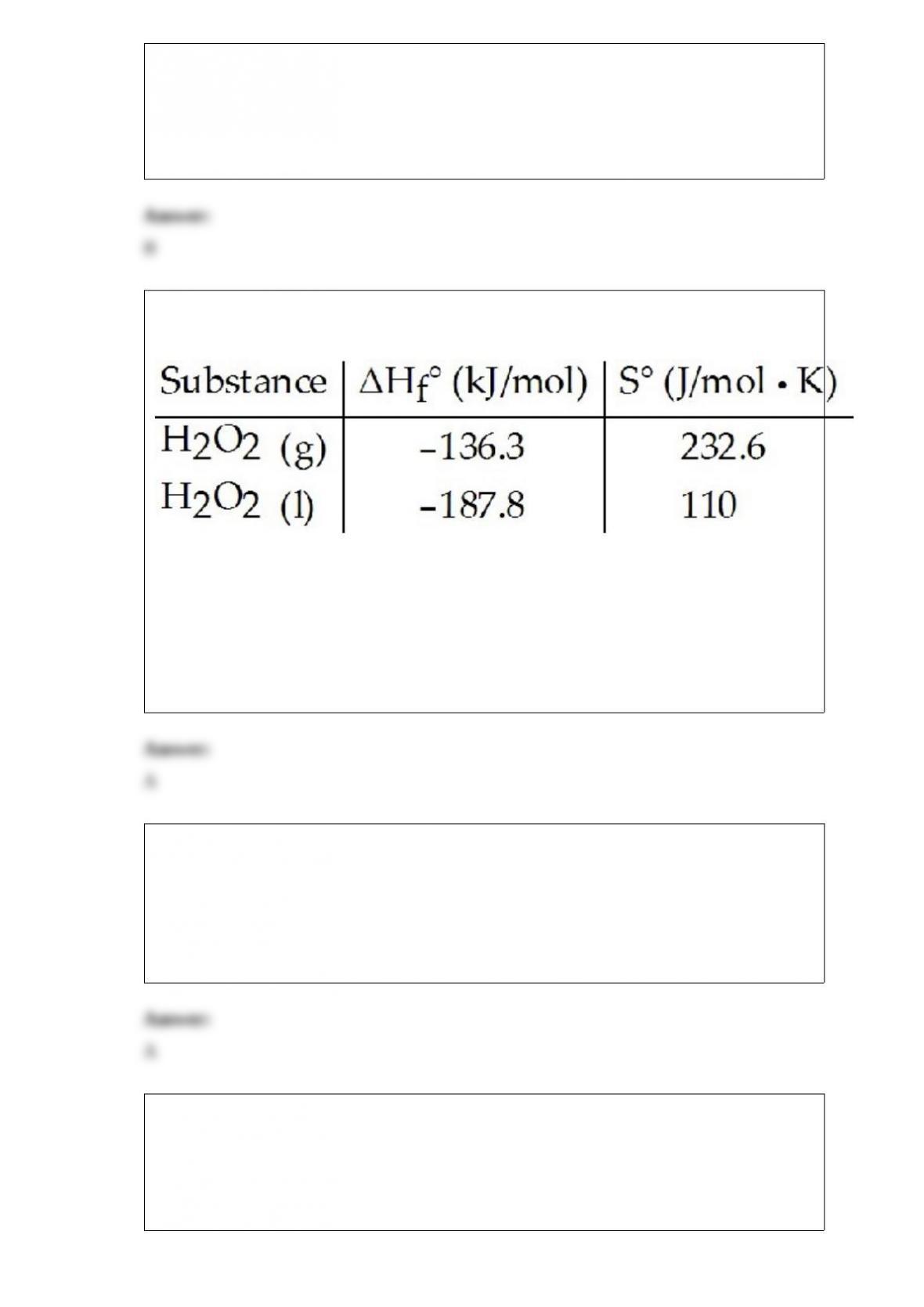
C) 3 NH3 and 1 Br-
D) 3 NH3, 1 Br-, and 1 H2O
E) 3 NH3, 2 Br-, and 1 H2O
9) In the presence of excess oxygen, methane gas burns in a constant-pressure system to
yield carbon dioxide and water:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)³ ΔH = -890.0 kJ
Calculate the value of q (kJ) in this exothermic reaction when 1.80 g of methane is
combusted at constant pressure.
A) -100.1 kJ
B) 0.0324 kJ
C) -0.0100 kJ
D) 30.9 kJ
E) -1.00 x 105 kJ
10) A result of the common-ion effect is ________.
A) that some ions, such as Na+ (aq), frequently appear in solutions but do not
participate in solubility equilibria
B) that common ions, such as Na+ (aq), don't affect equilibrium constants
C) that the selective precipitation of a metal ion, such as Ag+, is promoted by the
addition of an appropriate counterion (X-) that produces a compound (AgX) with a very
low solubility
D) that ions such as K+ and Na+ are common ions, so that their values in equilibrium
constant expressions are always 1.00
E) that common ions precipitate all counter-ions
11) An atom of 14C contains ________ electrons.
A) 14
B) 20
C) 8
D) 10
E) 6