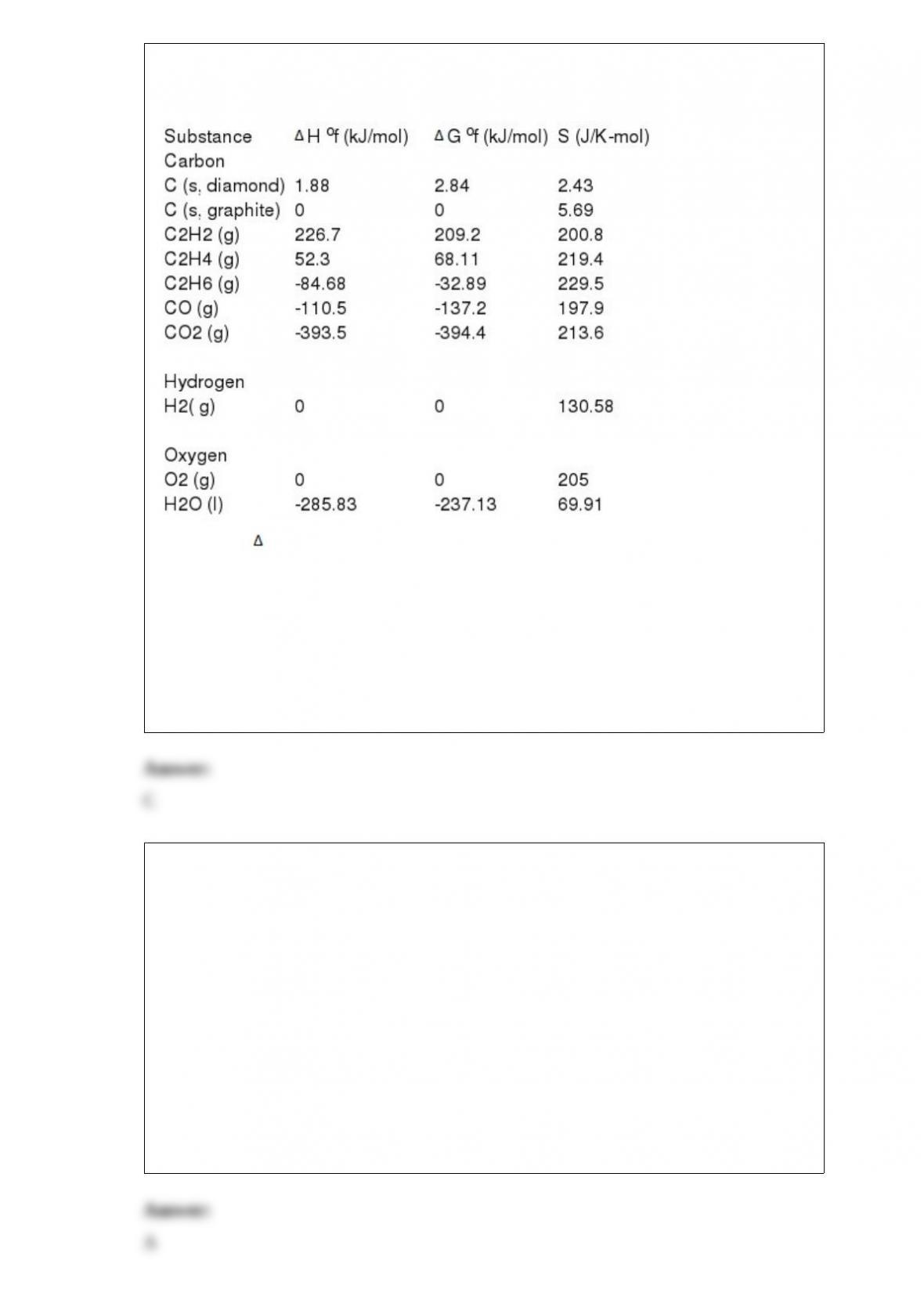
3) For a given reaction, H = +22.2 kJ/mol and S = +81.1 J/K-mol. The reaction is
spontaneous ________. Assume that H and S do not vary with temperature.
A) at T < 274 K
B) at T > 274 K
C) at T > 298 K
D) at T < 298 K
E) at all temperatures
4) The oxidation state of nitrogen in the NO2 molecule is ________.
A) +4
B) +3
C) +5
D) +2
E) -3
5) Based on electron configuration, which is most likely colorless?
A) [Cu(NH3)4]2+
B) [Cd(NH3)4]2+
C) [Ni(NH3)6]2+
D) [Cr(NH3)5Cl]2+
E) [Co(NH3)6]2+
6) You are given two clear solutions of the same unknown monoprotic acid, but with
different concentrations. Which statement is true?
A) There is no chemical method designed to tell the two solutions apart.
B) It would take more base solution (per milliliter of the unknown solution) to
neutralize the more concentrated solution.
C) A smaller volume of the less concentrated solution contains the same number of
moles of the acid compared to the more concentrated solution.
D) If the same volume of each sample was taken, then more base solution would be
required to neutralize the one with lower concentration.
E) The product of concentration and volume of the less concentrated solution equals the
product of concentration and volume of the more concentrated solution.