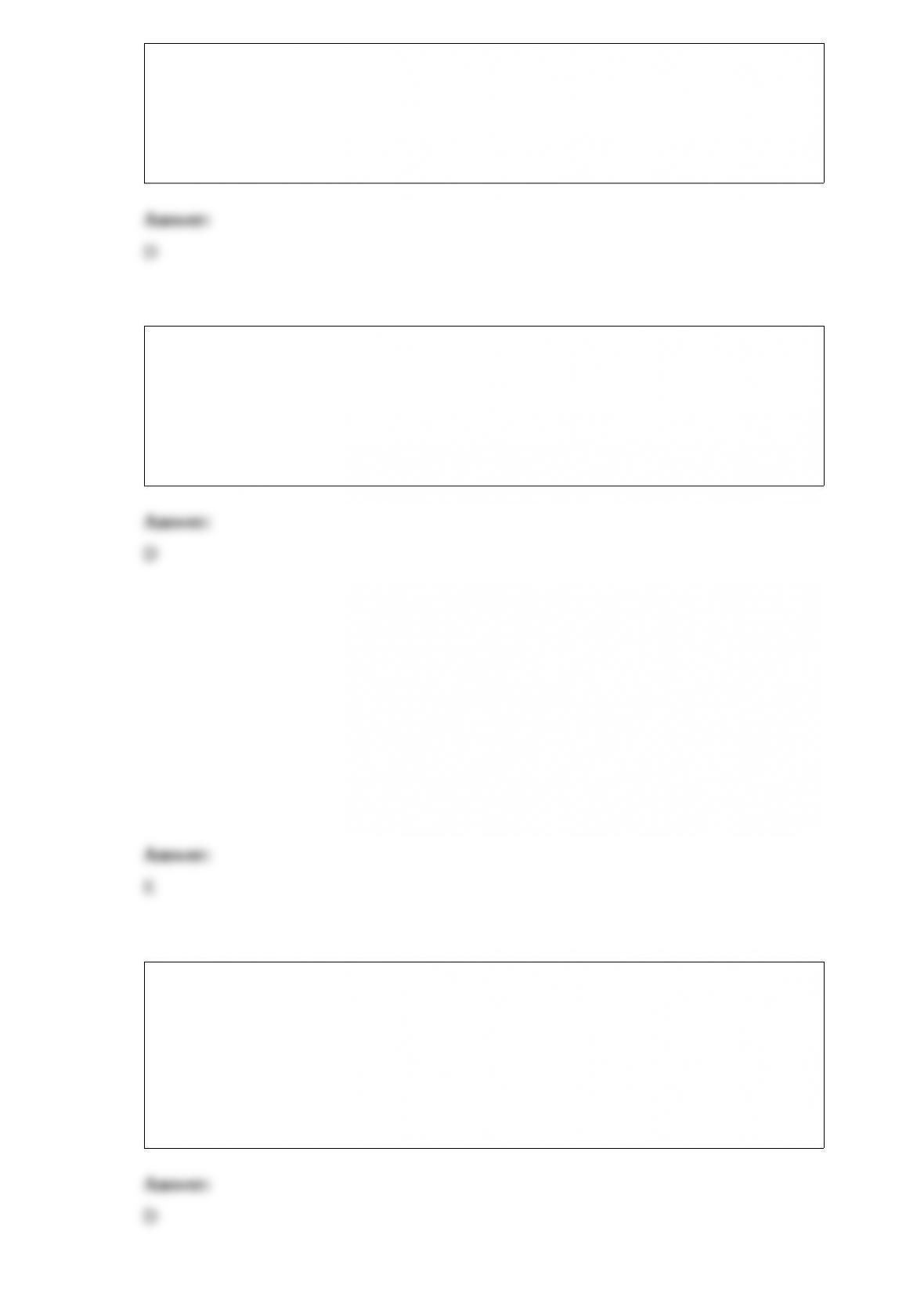
1) ________ is a unique element and does not truly belong to any family.
A) Nitrogen
B) Radium
C) Hydrogen
D) Uranium
E) Helium
2) The ________ ion has a noble gas electron configuration.
A) Be2+
B) Li2+
C) Li
D) Mg2-
E) Al2+
3) Which one of the choices below is not considered a fossil fuel?
A) anthracite coal
B) crude oil
C) natural gas
D) hydrogen
E) petroleum
4) (the first ionization energy ________.
A) decreases, decreasingly, increases
B) increases, increasingly, decreases
C) increases, increasingly, increases
D) decreases, increasingly, increases
E) decreases, increasingly, decreases
5) The central atom in the O3 molecule has ________ nonbonded electron pair(s) and
________ bonded electron pair(s) in its valence shell.
A) 6, 2
B) 1, 3