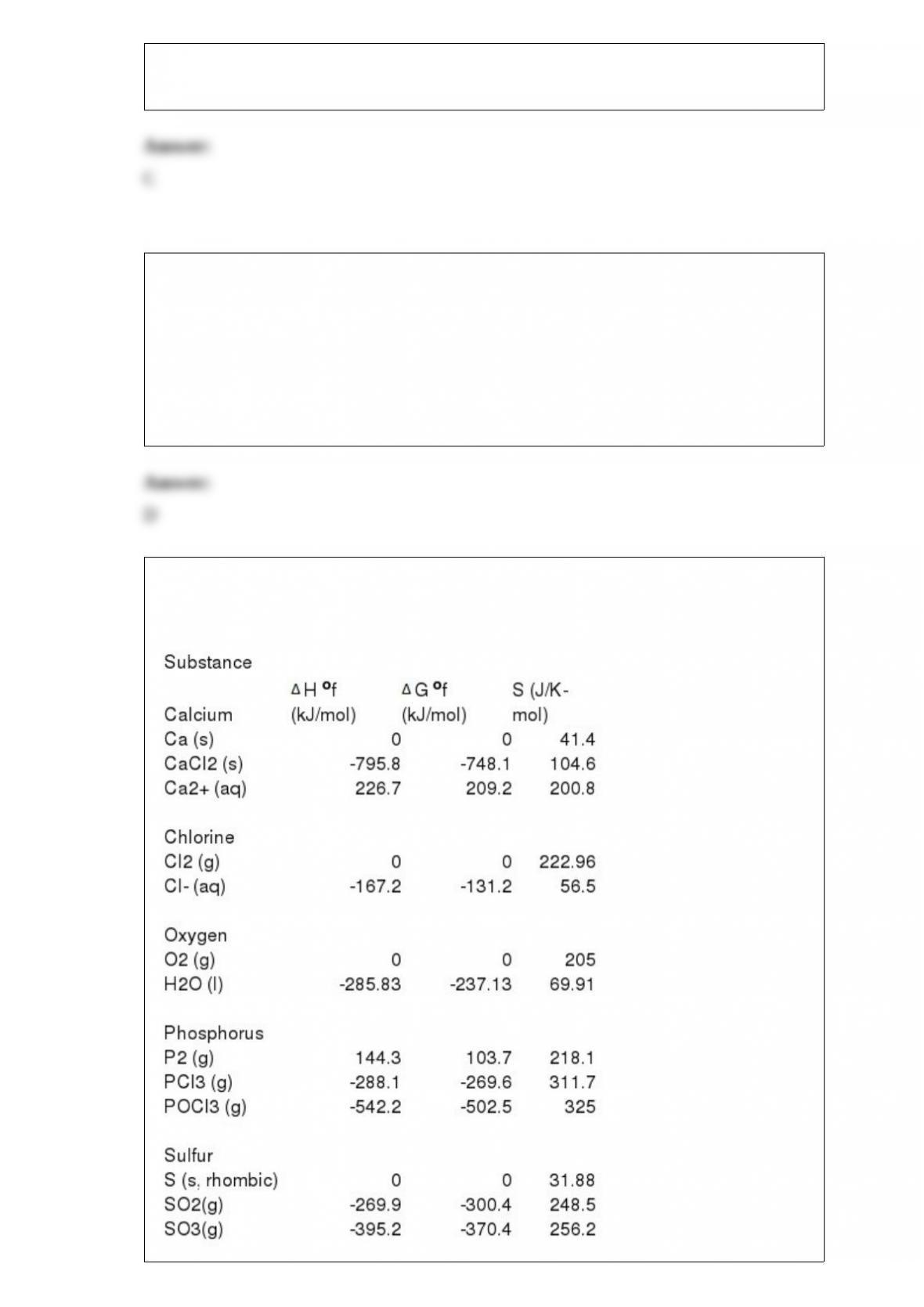
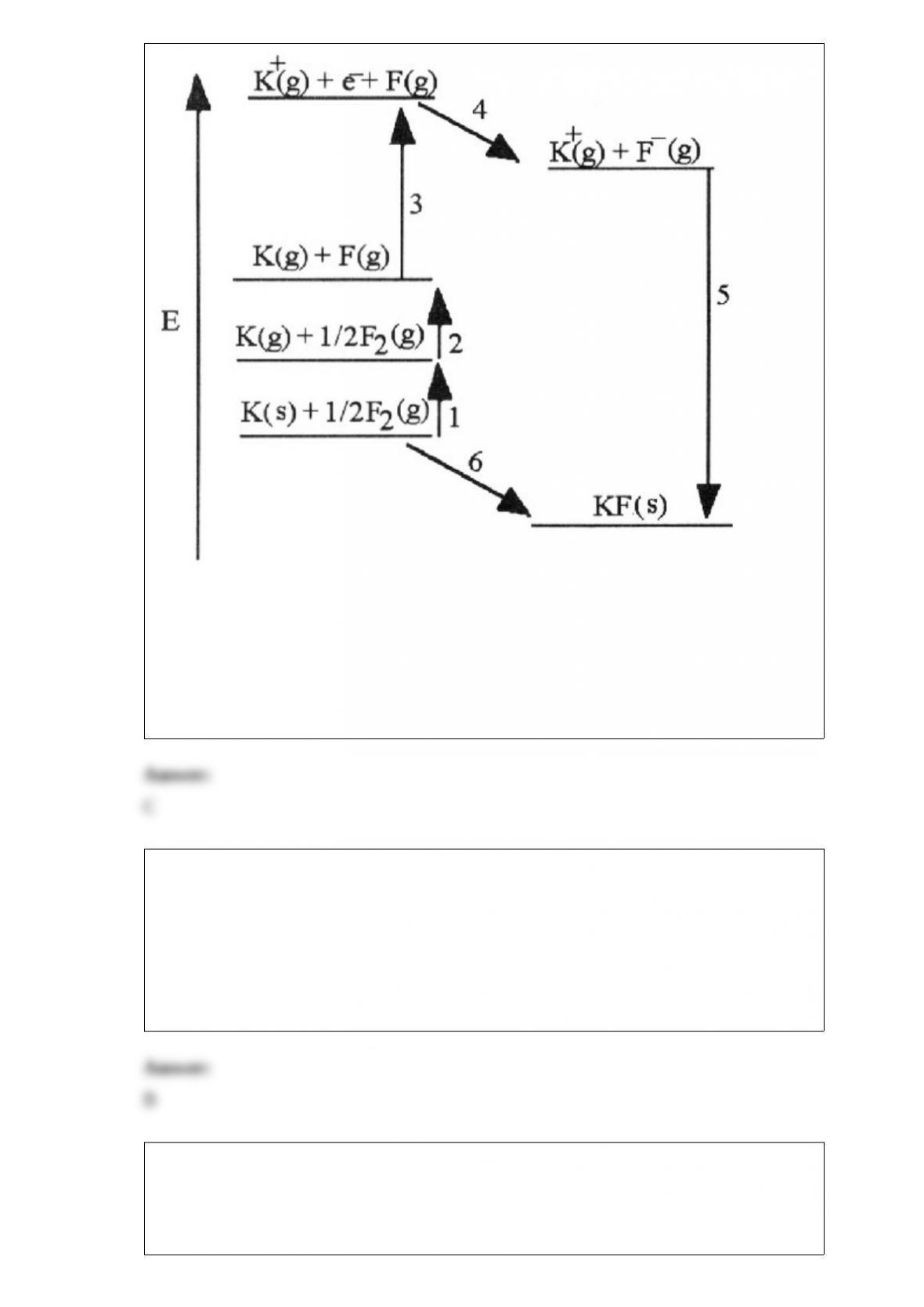
C) the light subatomic particles, protons and neutrons, reside in the nucleus
D) mass is spread essentially uniformly throughout the atom
E) the three principal subatomic particles (protons, neutrons, and electrons) all have
essentially the same mass and mass is spread essentially uniformly throughout the atom
12) Which of the following statements is false?
A) The change in entropy in a system depends on the initial and final states of the
system and the path taken from one state to the other.
B) Any irreversible process results in an overall increase in entropy.
C) The total entropy of the universe increases in any spontaneous process.
D) Entropy increases with the number of microstates of the system.
13) The total number of orbitals in a shell is given by ________.
A) I2
B) n2
C) 2n
D) 2n + 1
E) 2l + 1