Which of the following unit factors is incorrect?
A)1 microliter/1000 nL C)1 L/1000 mL
B)1 cg/100 g D)1000 m/1 km
Which statement is true?
A) All real processes are irreversible.
B) A thermodynamically reversible process takes place infinitely fast.
C) In a reversible process, the state functions of the system are always much greater
than those of the surroundings.
D) There is always more heat given off to the surroundings in a reversible process than
in an unharnessed one.
E) All statements (A-D) are true.
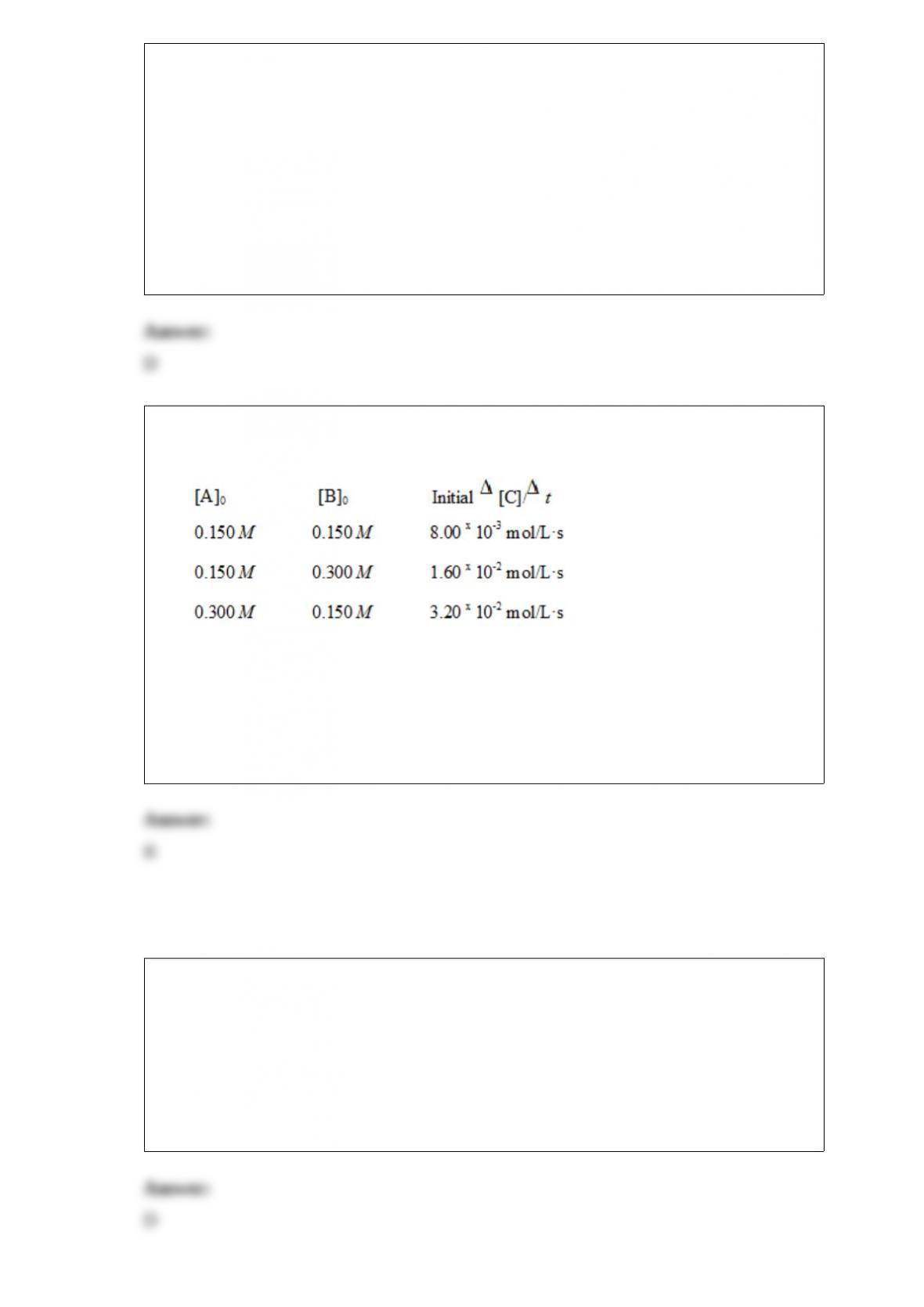
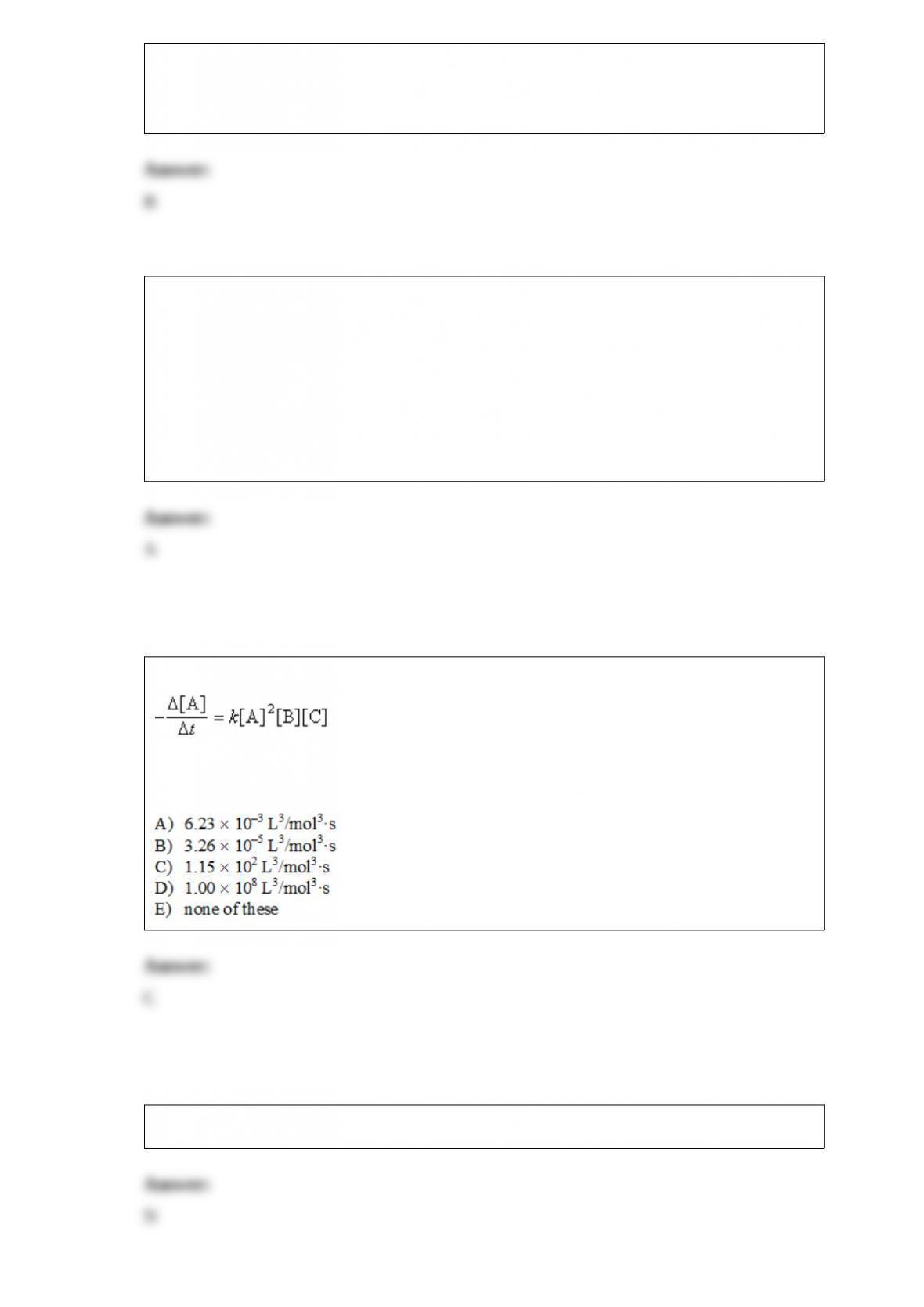
Consider the reaction 3A + B + C --> D + E where the rate law is defined as
.
An experiment is carried out where [B]0= [C]0= 1.00 M and [A]0= 1.00 x10-4M.
After 3.00 minutes, [A] = 3.26 x10-5M. The value of k is
Larger first ionization energy, C or N