9) The net ionic equation for the reaction between aqueous nitric acid and aqueous
sodium hydroxide is ________.
A) H+ (aq) + HNO3 (aq) + 2OH- (aq) → 2H2O (l) + NO3
- (aq)
B) HNO3 (aq) + NaOH (aq) → NaNO3 (aq) + H2O (l)
C) H+ (aq) + OH- (aq) → H2O (l)
D) HNO3 (aq) + OH- (aq) → NO3
- (aq) + H2O (l)
E) H+ (aq) + Na+ (aq) + OH- (aq) → H2O (l) + Na+ (aq)
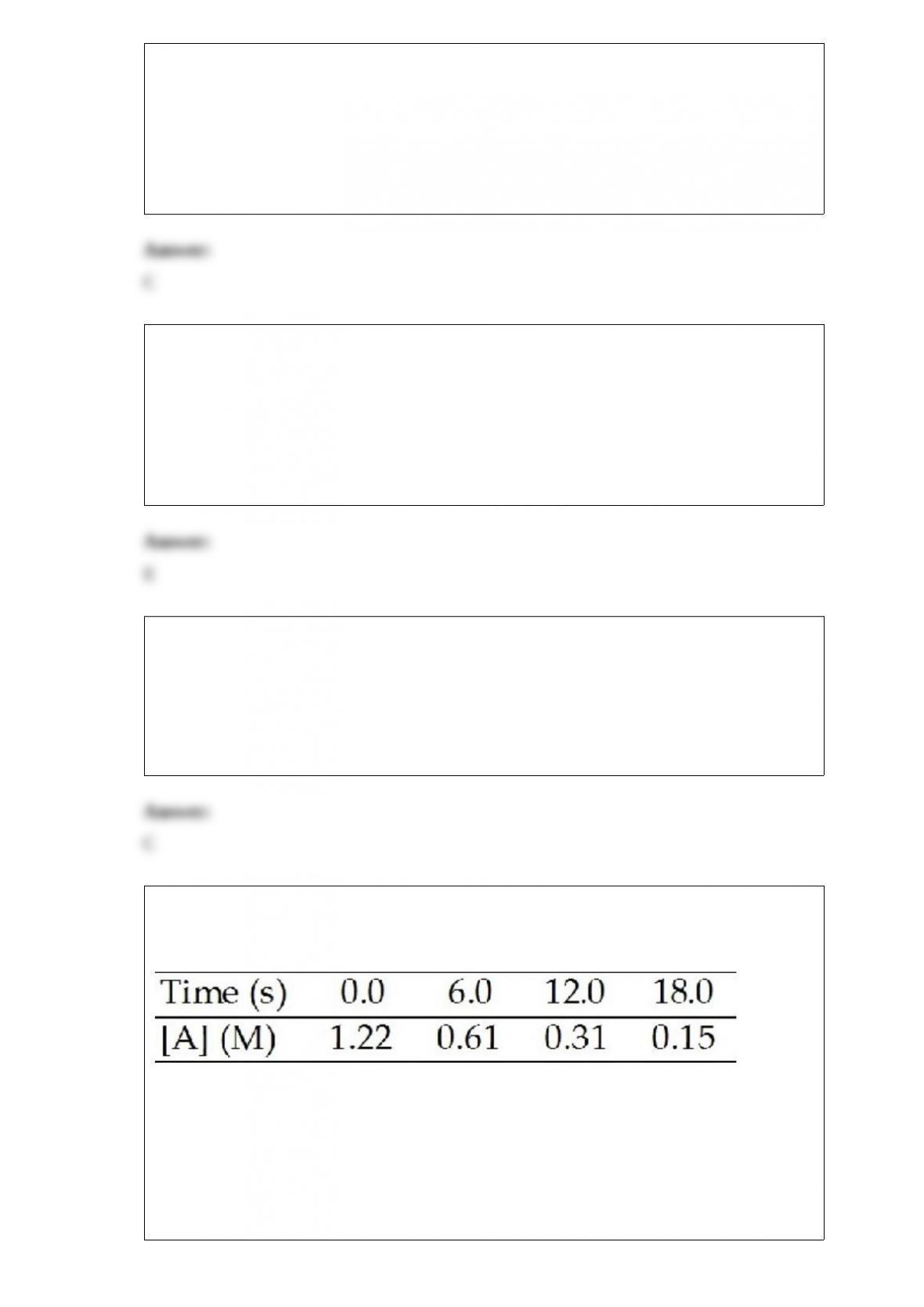
10) A compound decomposes by a first-order process. If 17.0% of the compound
decomposes in 30 minutes, the half-life of the compound is ________.
A) 112 minutes
B) 12 minutes
C) 8 minutes
D) 223 minutes
E) 56 minutes
11) Cathode rays are deflected away from a negatively charged plate because
________.
A) they are not particles
B) they are positively charged particles
C) they are neutral particles
D) they are negatively charged particles
E) they are emitted by all matter
12) In order to produce sp2 hybrid orbitals, ________ s atomic orbital(s) and ________
p atomic orbital(s) must be mixed.
A) one, two
B) one, three
C) one, one
D) two, two