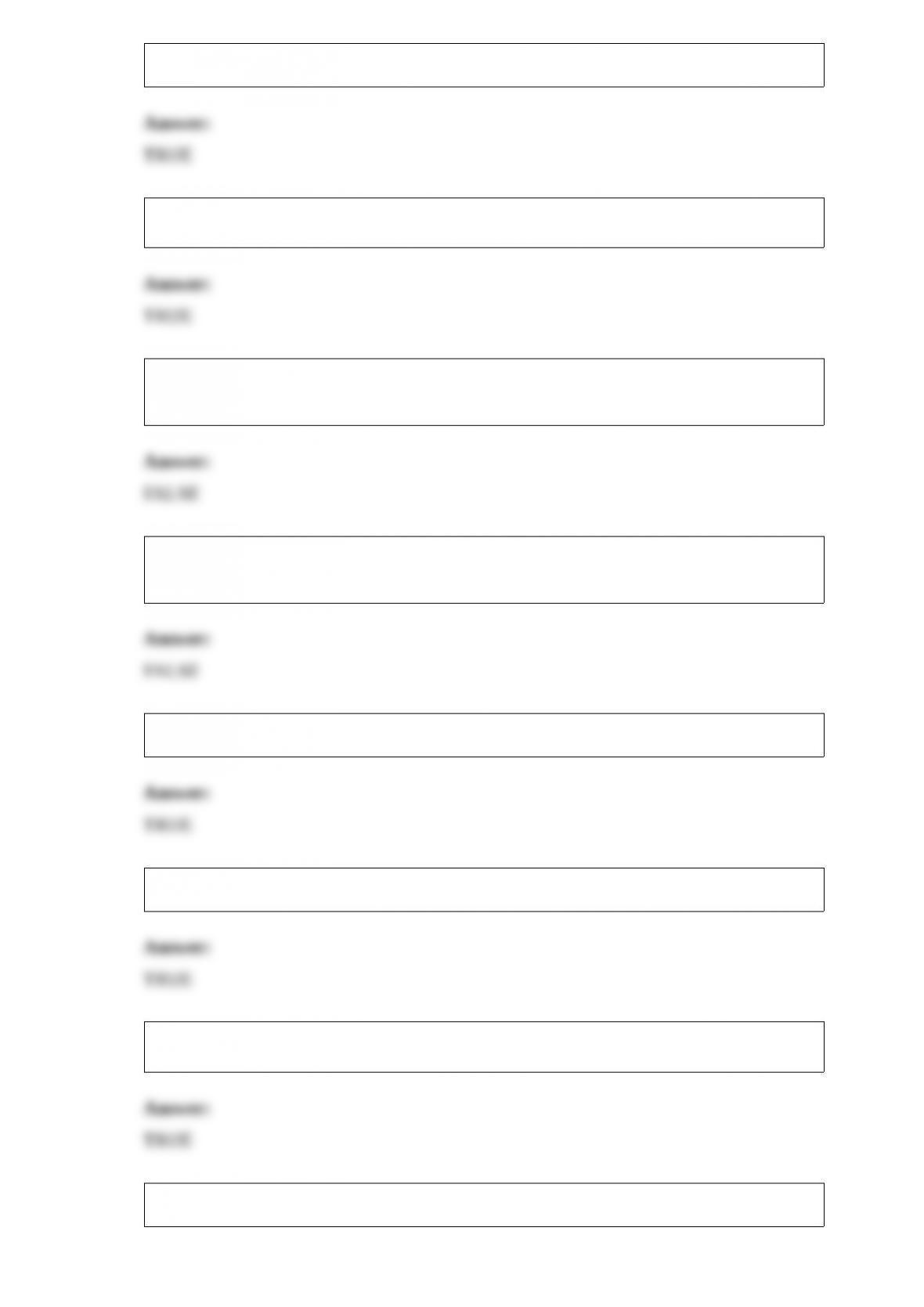
13) The empirical formula of a compound with molecules containing 12 carbon atoms,
14 hydrogen atoms, and 6 oxygen atoms is ________.
A) C12H14O6
B) CHO
C) CH2O
D) C6H7O3
E) C2H4O
14) Which combination will produce a precipitate?
A) NaC2H3O2 (aq) and HCl (aq)
B) NaOH (aq) and HCl (aq)
C) AgNO3(aq) and Ca(C2H3O2)2 (aq)
D) KOH (aq) and Mg(NO3)2 (aq)
E) NaF (aq) and HCl (aq)
15) The name of the ionic compound NH4CN is ________.
A) nitrogen hydrogen cyanate
B) ammonium carbonitride
C) ammonium cyanide
D) ammonium hydrogen cyanate
E) cyanonitride
16) A solution is prepared by dissolving 0.60 g of nicotine (a nonelectrolyte) in water to
make 12 mL of solution. The osmotic pressure of the solution is 7.55 atm at 25 oC. The
molecular weight of nicotine is ________ g/mol.
A) 28
B) 43
C) 50
D) 160
E) 0.60