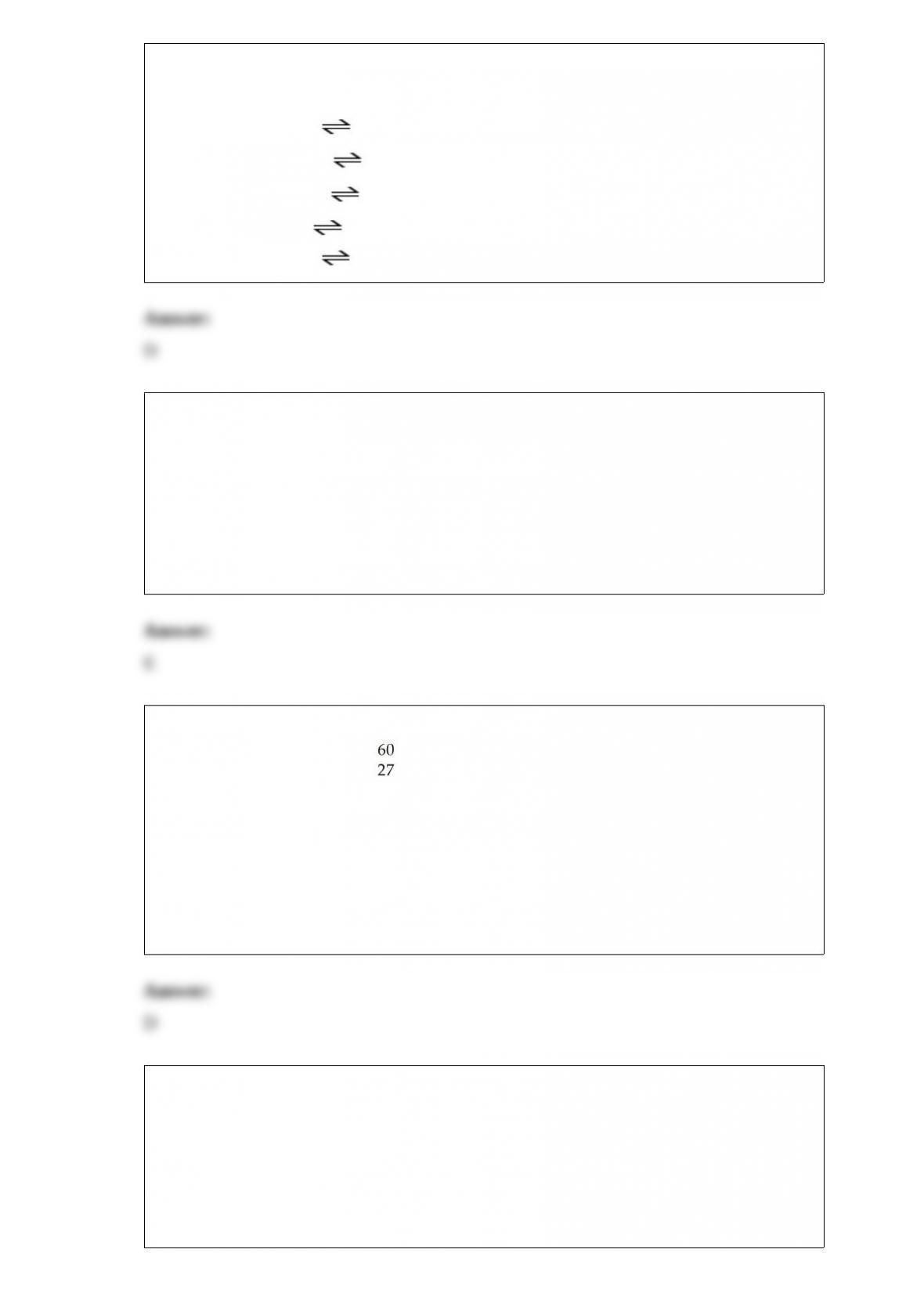
1) HA is a weak acid. Which equilibrium corresponds to the equilibrium constant Kb for
A-?
A) HA (aq) + H2O (l) H2A+ (aq) + OH-(aq)
B) A- (aq) + H3O+ (aq) HA (aq) + H2O (l)
C) HA (aq) + OH- (aq) H2O (l) + H+ (aq)
D) A- (aq) + H2O (l) HA (aq) + OH- (aq)
E) A- (aq) + OH- (aq) HOA2- (aq)
2) Complexes containing metals with which one of the following electron
configurations are usually colorless?
A) d2
B) d1
C) d5
D) d8
E) d10
3) The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is
the binding energy (in J) of a Co nucleus? (The mass of a cobalt-60 nucleus is
59.9338 amu.)
A) 2.74 x 10-19
B) 9.12 x 10-28
C) 4.94 x 10-13
D) 8.20 x 10-11
E) 2.74 x 10-16
4) In the smectic A liquid-crystalline phase, ________.
A) the molecules are aligned along their long axes, with no ordering with respect to the
ends of the molecules
B) the molecules are arranged in sheets, with their long axes parallel and their ends
aligned as well
C) the molecules are aligned with their long axes tilted with respect to a line
perpendicular to the plane in which the molecules are stacked