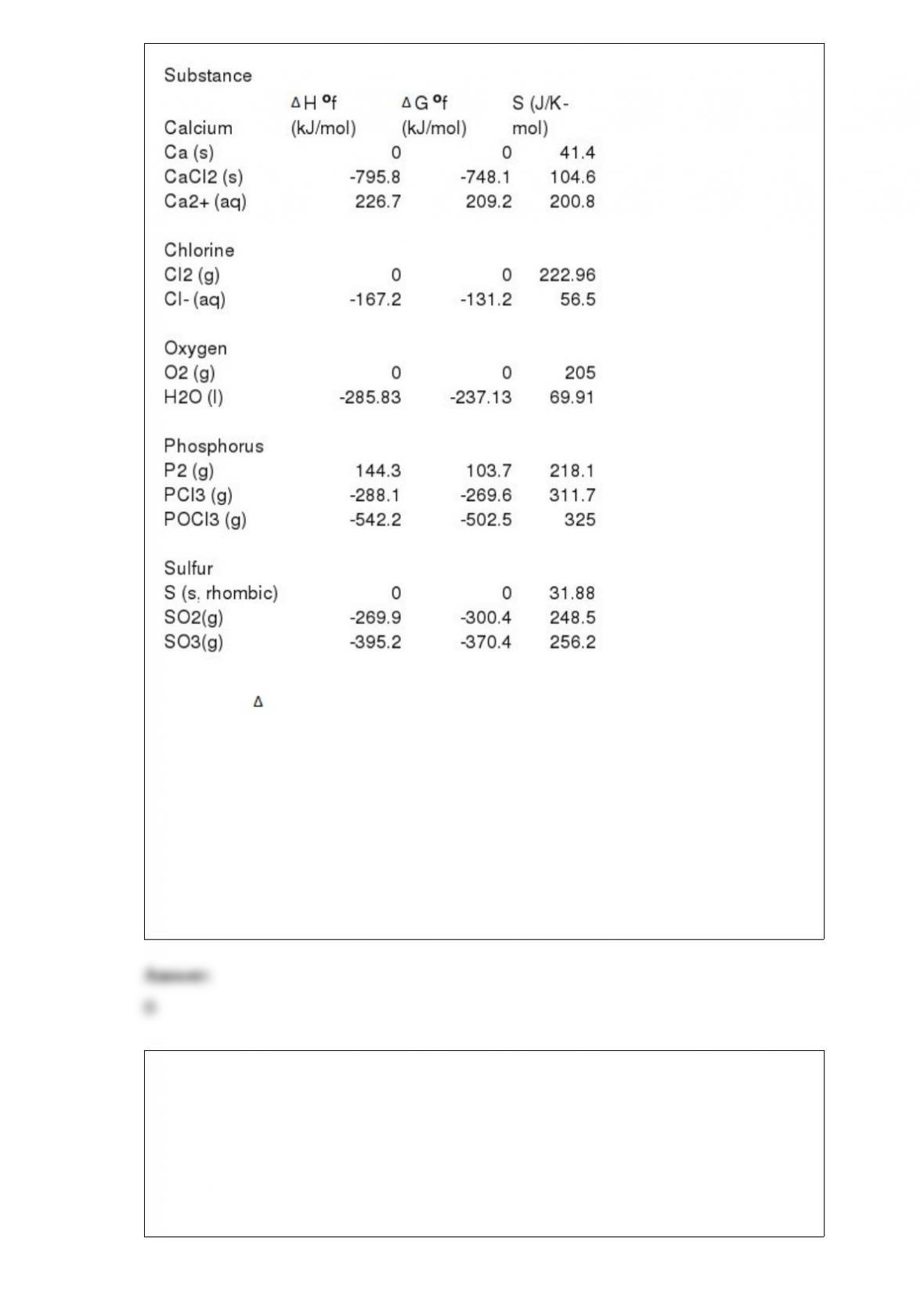
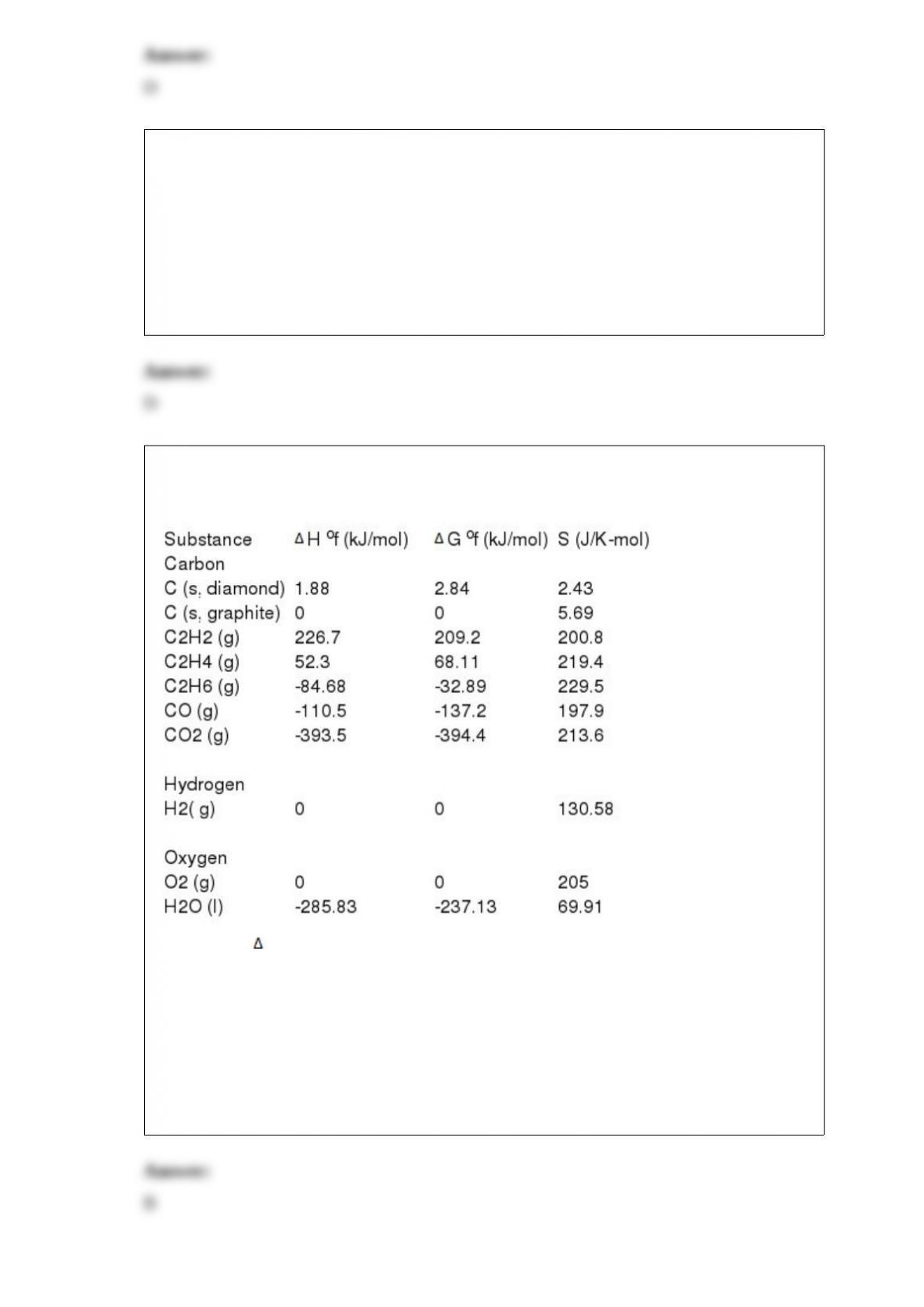
1) The specific heat capacity of lead is 0.13 J/g-K. How much heat (in J) is required to
raise the temperature of 15 g of lead from 22 °C to 37 °C?
A) 2.0 J
B) -0.13 J
C) 5.8 x 10-4 J
D) 29 J
E) 0.13 J
2) Carbon dioxide is produced ________.
A) in blast furnaces when metal oxides are reduced with CO
B) by combustion of carbon-containing substances in an excess of oxygen
C) when carbonates are heated
D) by fermentation of sugar during the production of ethanol
E) by all of the above processes
3) A solution is prepared by mixing 15.0 mL of 0.100 M HCl and 5.00 mL of 0.200 M
NaCl. What is the molarity of chloride ion in this solution?
A) 0.175
B) 8.00
C) 1.25
D) 0.0250
E) 0.125
4) What happens to the mass number and the atomic number of an element when it
undergoes alpha decay?
A) The mass number decreases by 4 and the atomic number decreases by 2
B) The mass number does not change and the atomic number increases by 1
C) The mass number increases by 4 and the atomic number does not change.
D) The mass number increases by 2 and the atomic number decreases by 1
E) The mass number does not change and the atomic number increases by 2
5) Which noble gas is expected to show the largest deviations from the ideal gas