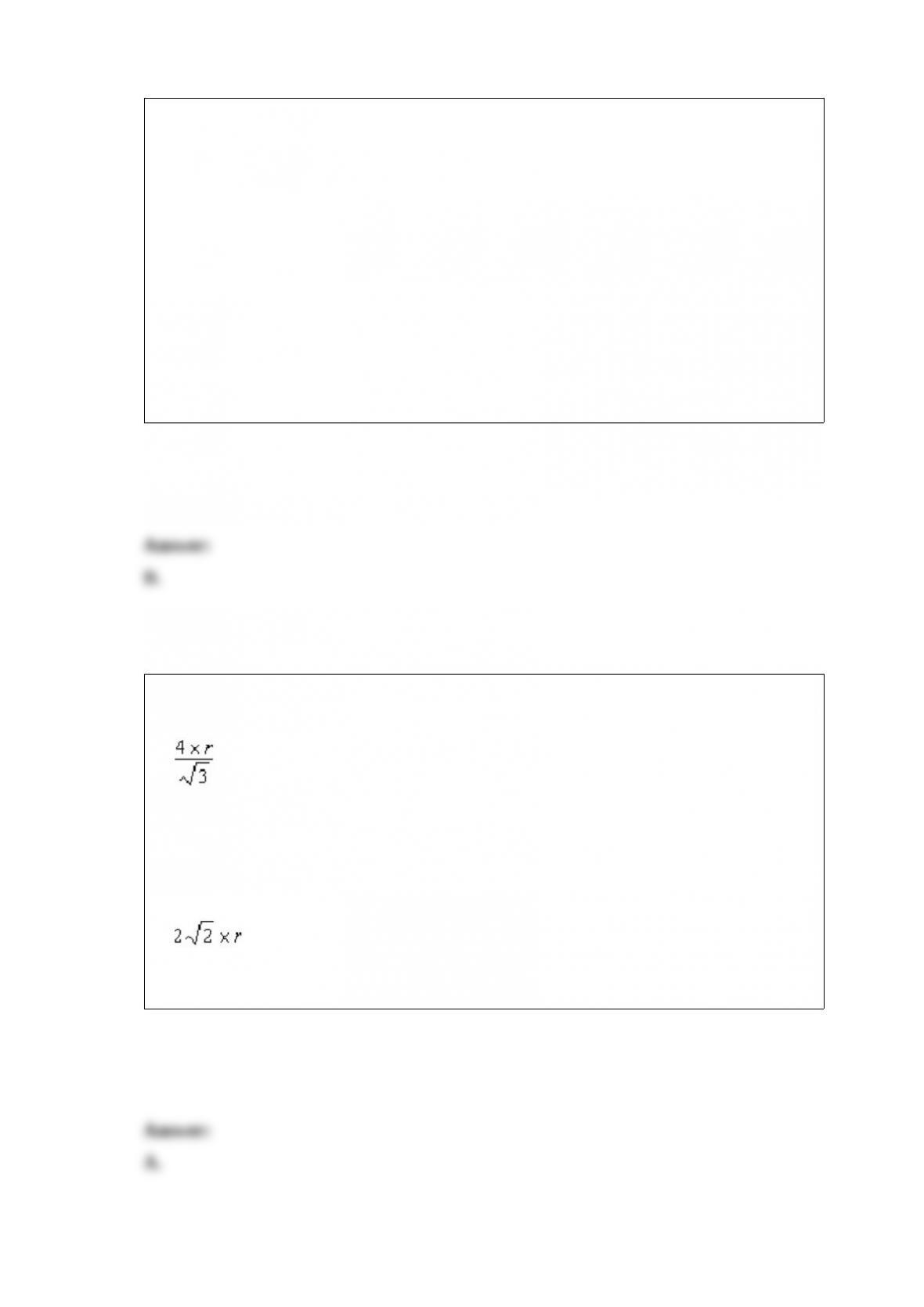
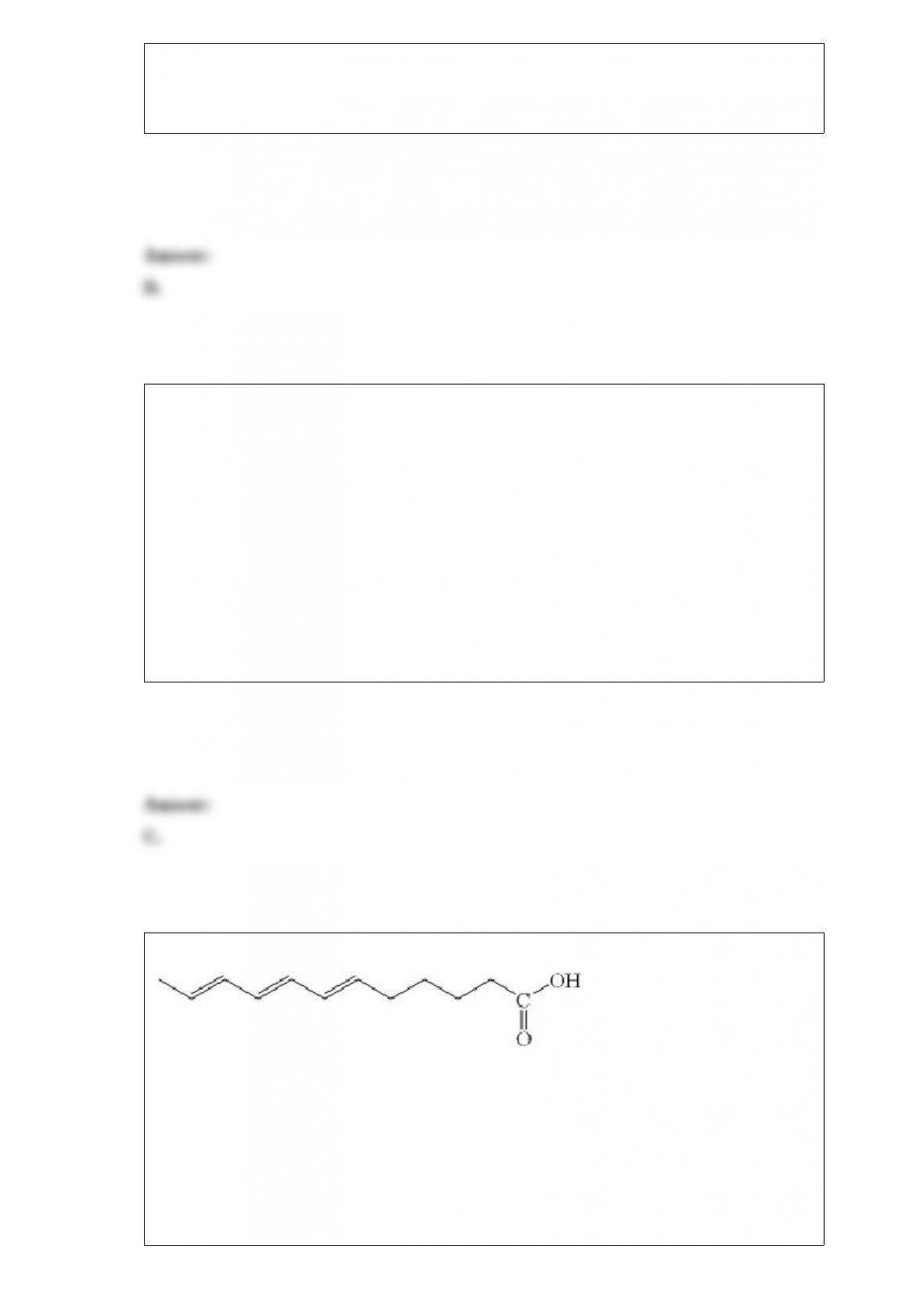
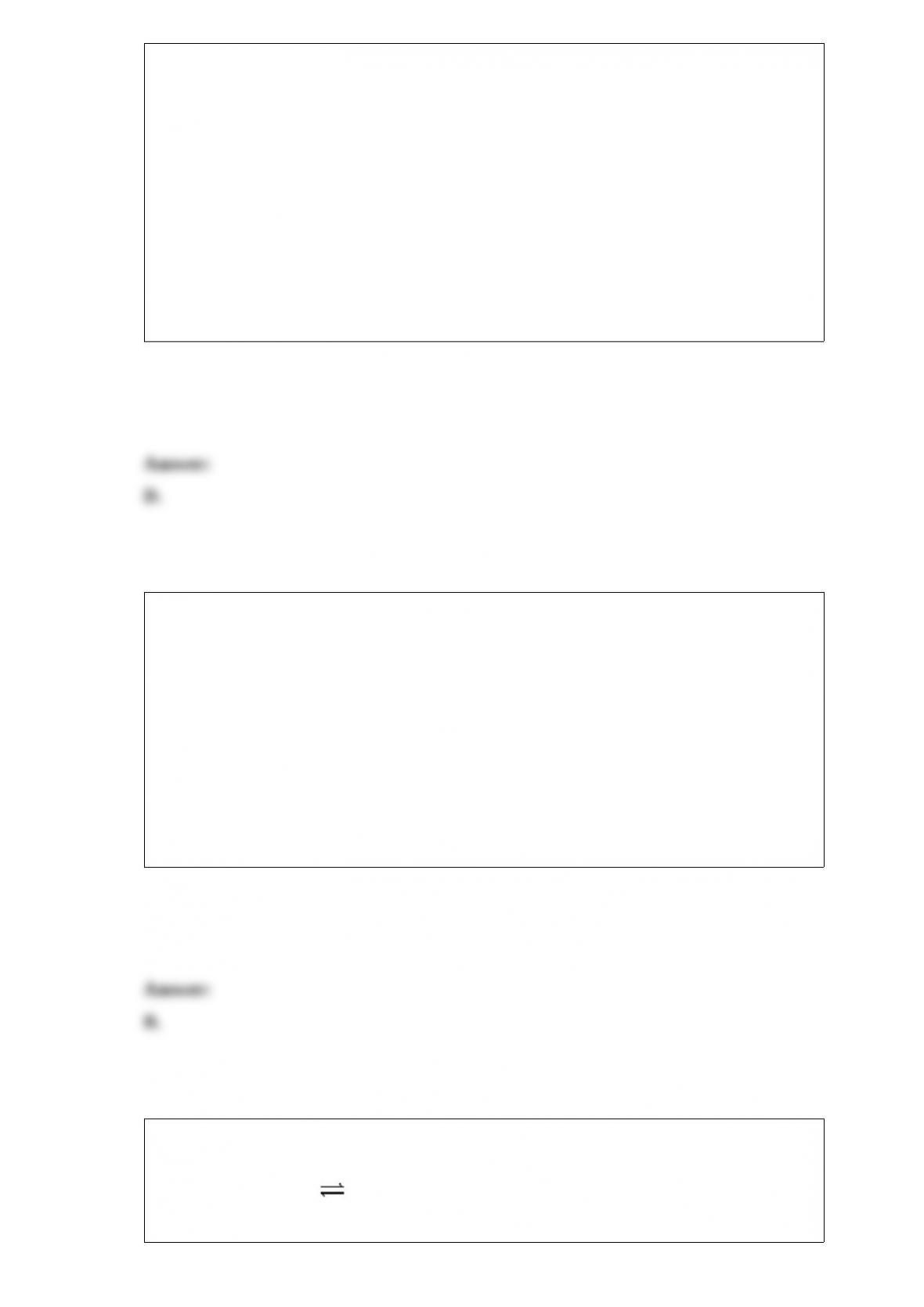
What is the correct cell notation for a voltaic cell based on the reaction below?
Cu2+(aq) + Fe(s) Cu(s) + Fe2+(aq)
A.Cu(s) | Cu2+(aq) || Fe2+(aq) | Fe(s)
B.Fe(s) || Fe2+(aq), Cu2+(aq) | Cu(s)
C.Cu(s) || Cu2+(aq), Fe2+(aq) || Fe(s)
D.Cu(s) | Fe2+(aq) || Cu2+(aq) | Fe(s)
E.Fe(s) | Fe2+(aq) || Cu2+(aq) | Cu(s)
What is the product of the addition of excess F2 to acetylene?
A.1,2-difluoroethane
B.ethylene
C.1,1,2,2-tetrafluoroethane
D.ethane
E.1,1-difluoroethylene
Which of the following statements is/are CORRECT?