29) Given the equation:
C2H6 (g) + O2 (g) → CO2 (g) + H2O (g) (not balanced)
Determine the number of liters of O2 consumed at STP when 60.0 grams of C2H6 is
burned.
30) Briefly explain why carbon and silicon can form oxides with such different physical
properties, gaseous CO2and solid SiO2.
31) The correct answer (reported to the proper number of significant figures) to the
following is ________.
(1501-1496) X (9.18 X 3.68) = ________
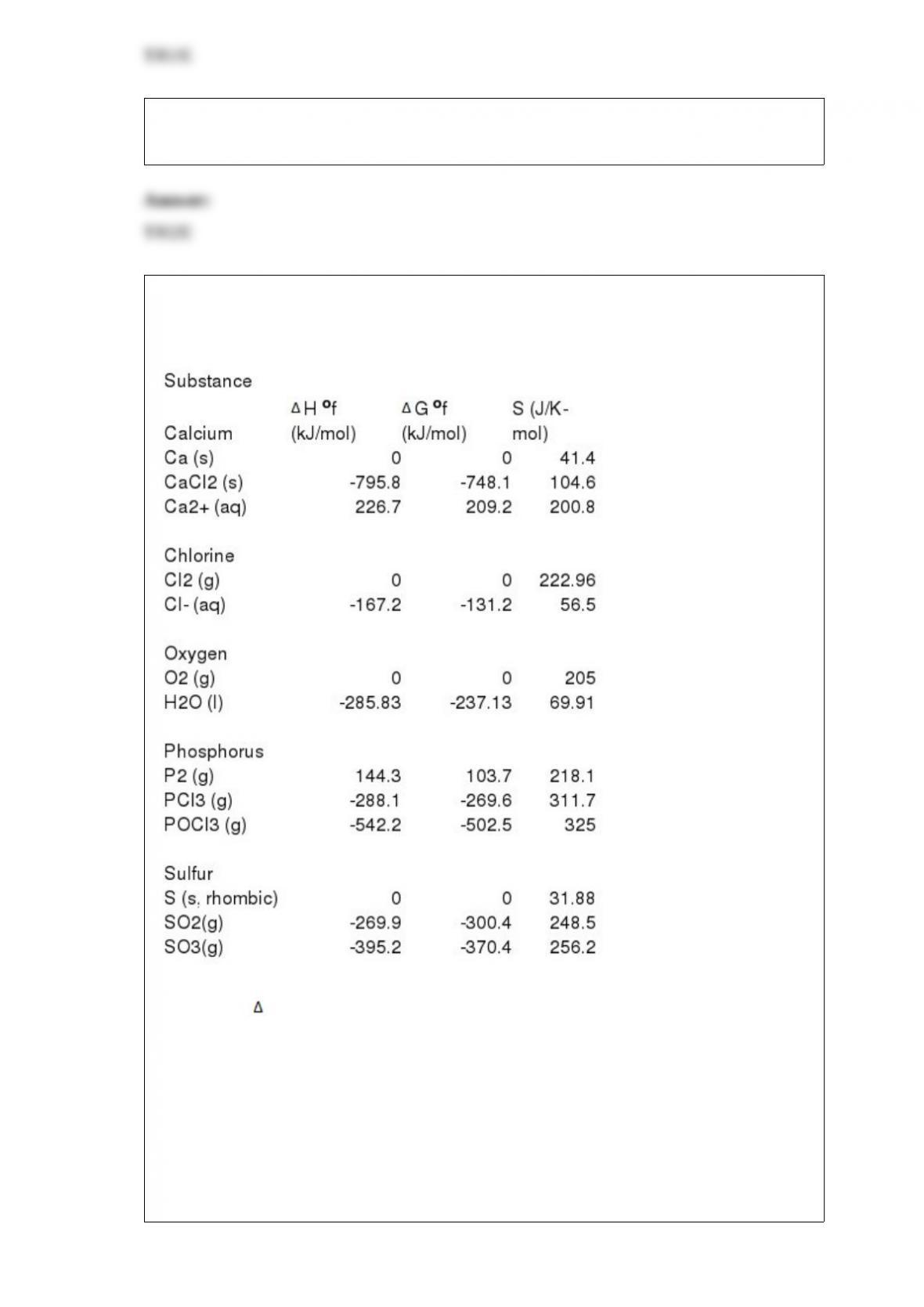
32) Calculate G o(in kJ/mol) for the following reaction at 1 atm and 25 oC:
C2H6 (g) + O2 (g) --> CO2 (g) + H2O (l) (unbalanced)
Gf
o C2H6 (g) = -32.89 kJ/mol; Gf
oCO2 (g) = -394.4 kJ/mol; Gf
o H2O (l) = -237.13
kJ/mol
33) The sensation of vision results from a nerve impulse that is triggered by the
separation of retinal from ________.