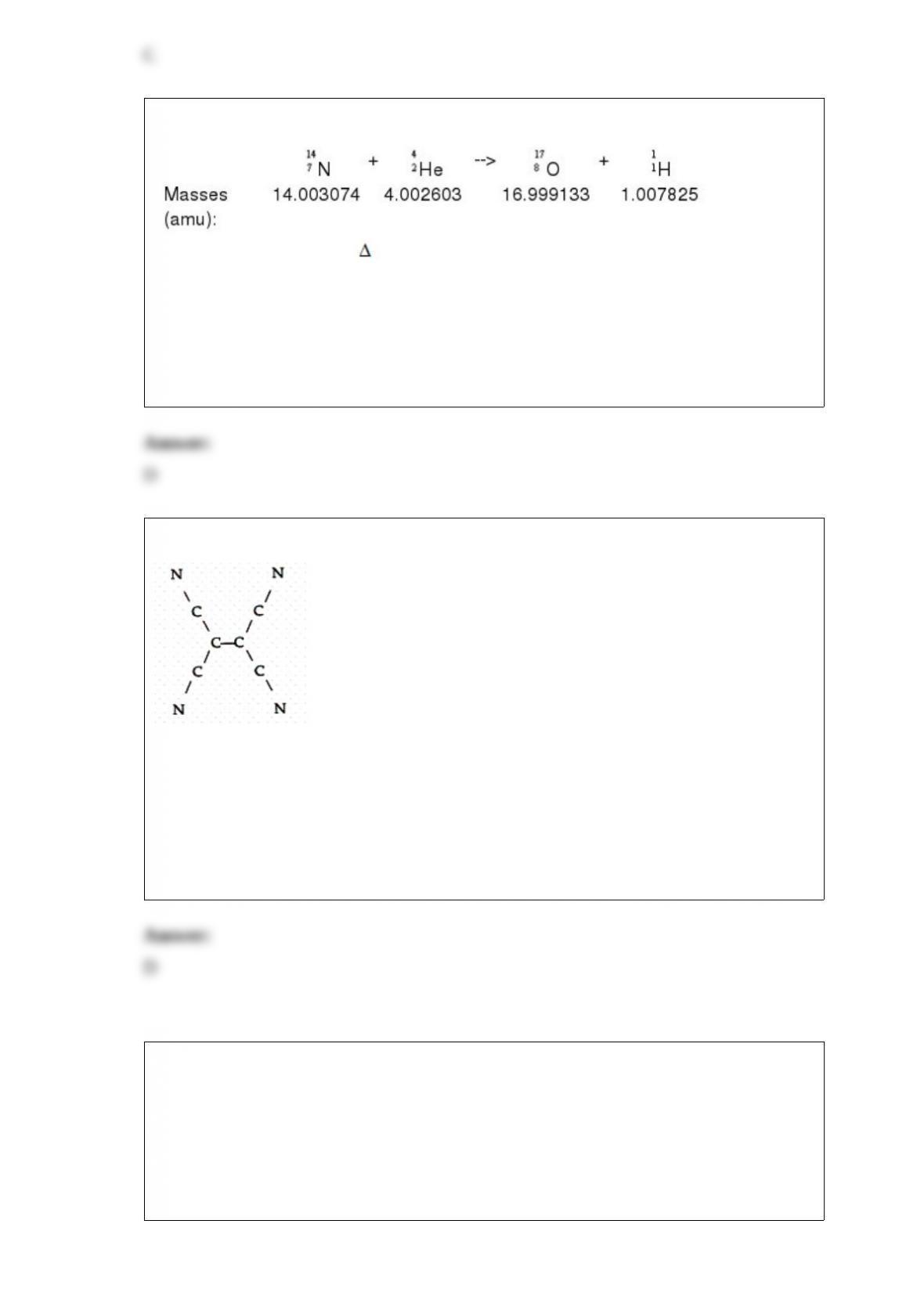
a)changing the volume at constant temperature?
b)increasing the temperature at constant volume
A) a) equilibrium shifts towards products, b) equilibrium shifts towards products.
B) a) equilibrium shifts towards reactants, b) equilibrium shifts towards products.
C) a) equilibrium shifts towards products, b) equilibrium shifts towards reactants.
D) a) no change in the equilibrium, b) equilibrium shifts towards products.
E) a) no change in the equilibrium, b) equilibrium shifts towards reactants.
Which of the following ions forms the fewest insoluble salts?
A)Al3+
B)Cl-
C)NO3
-
D)OH-
E)Mg2+
When the volume is decreased at constant temperature, the ratio of the partial pressure
of PCl5to the partial pressure of PCl3will
A) increase
B) decrease
C) stay the same
D) impossible to tell without more information
E) none of these
Which one of the following statements is false?
A)The change in internal energy, DE, for a process is equal to the amount of heat
absorbed at constant volume, qv.
B)The change in enthalpy, DH, for a process is equal to the amount of heat absorbed at
constant pressure, qp.
C)A bomb calorimeter measures DH directly.
D)If qpfor a process is negative, the process is exothermic.
E)The freezing of water is an example of an exothermic reaction.