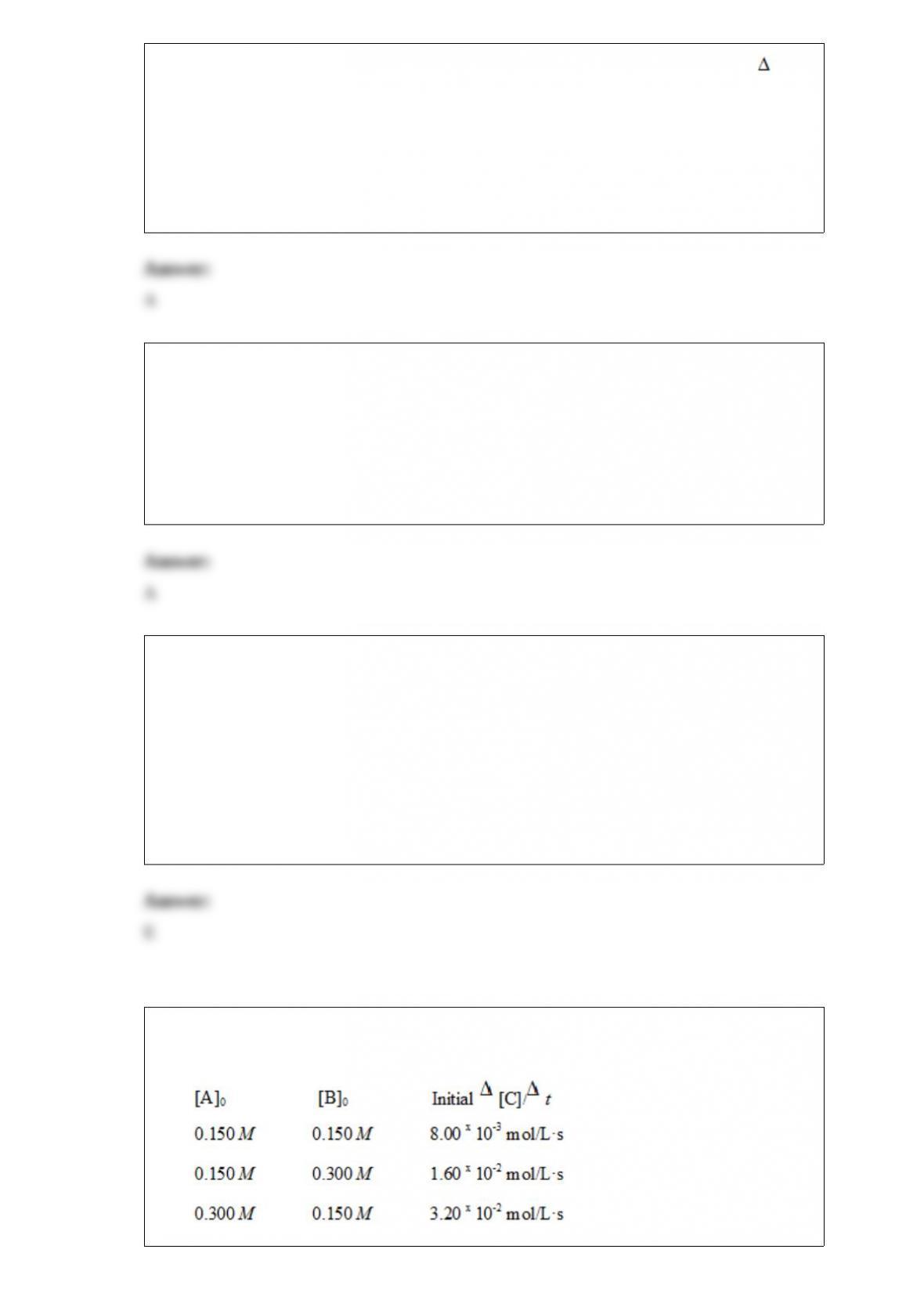
What is the overall order of the reaction?
A)0
B)1
C)2
D)3
E)4
Which of the following elements has the highest density?
A) Hg
B) W
C) Ta
D) Au
E) Ir
The statement that the first ionization energy for an oxygen atom is lower than the first
ionization energy for a nitrogen atom is
A)consistent with the general trend relating changes in ionization energy across a period
from left to right, because it is easier to take an electron from an oxygen atom than from
a nitrogen atom
B)consistent with the general trend relating changes in ionization energy across a period
from left to right, because it is harder to take an electron from an oxygen atom than
from a nitrogen atom
C)inconsistent with the general trend relating changes in ionization energy across a
period from left to right, due to the fact that the oxygen atom has two doubly-occupied
2p orbitals and nitrogen has only one
D)inconsistent with the general trend relating changes in ionization energy across a
period from left to right, due to the fact that oxygen has one doubly-occupied 2p orbital
and nitrogen does not
E)incorrect
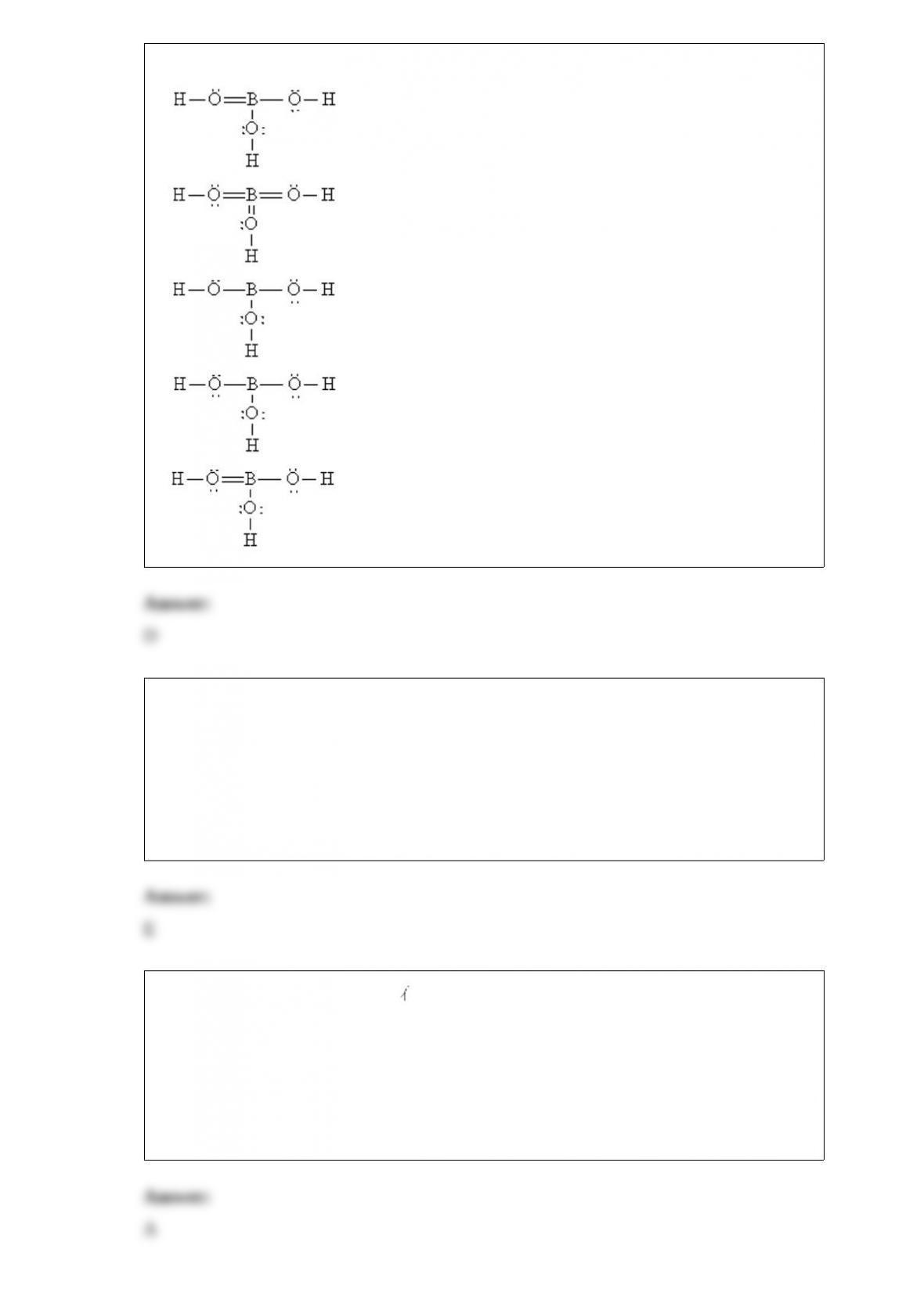
Consider the molecule and the following hybridization choices: