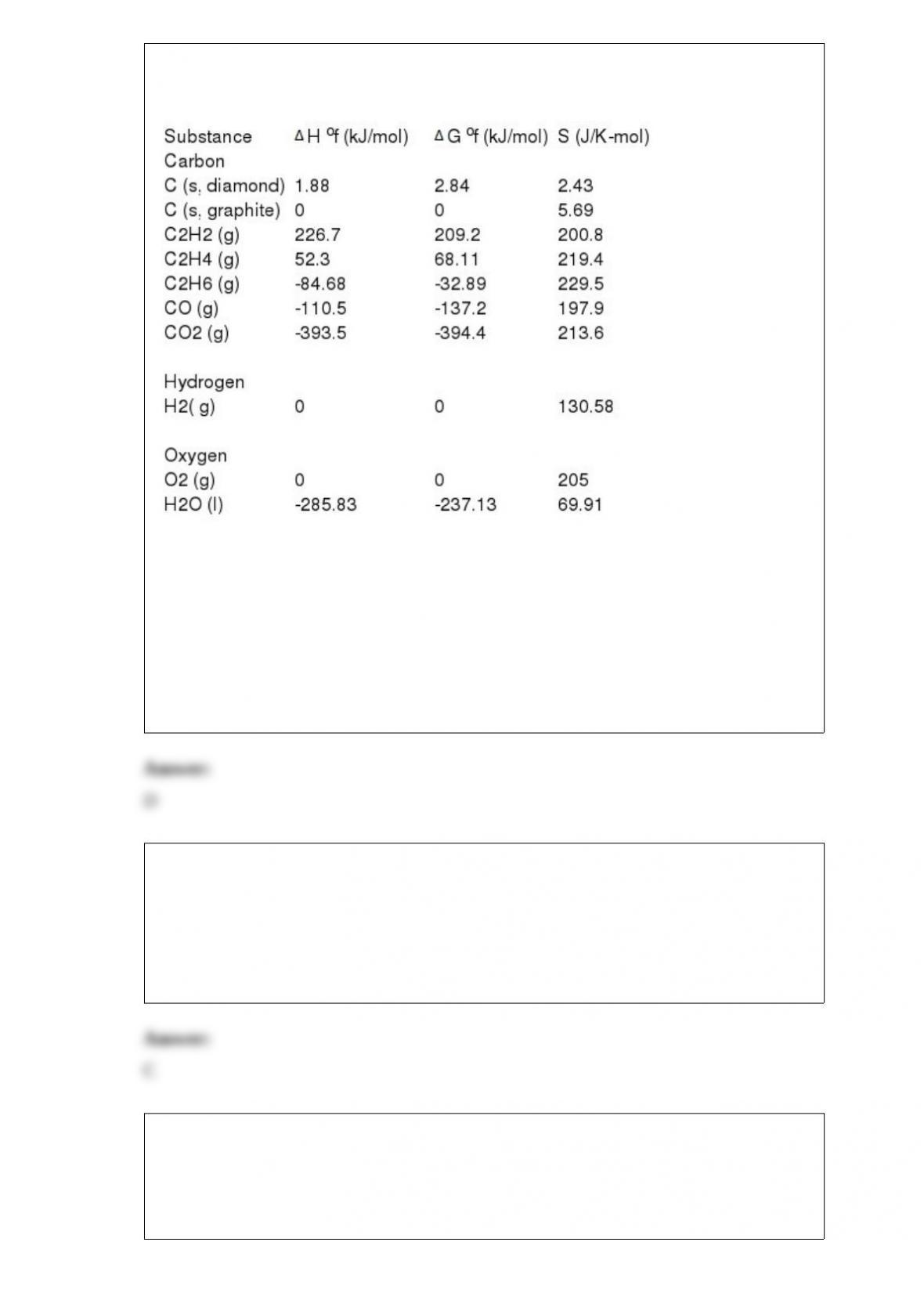
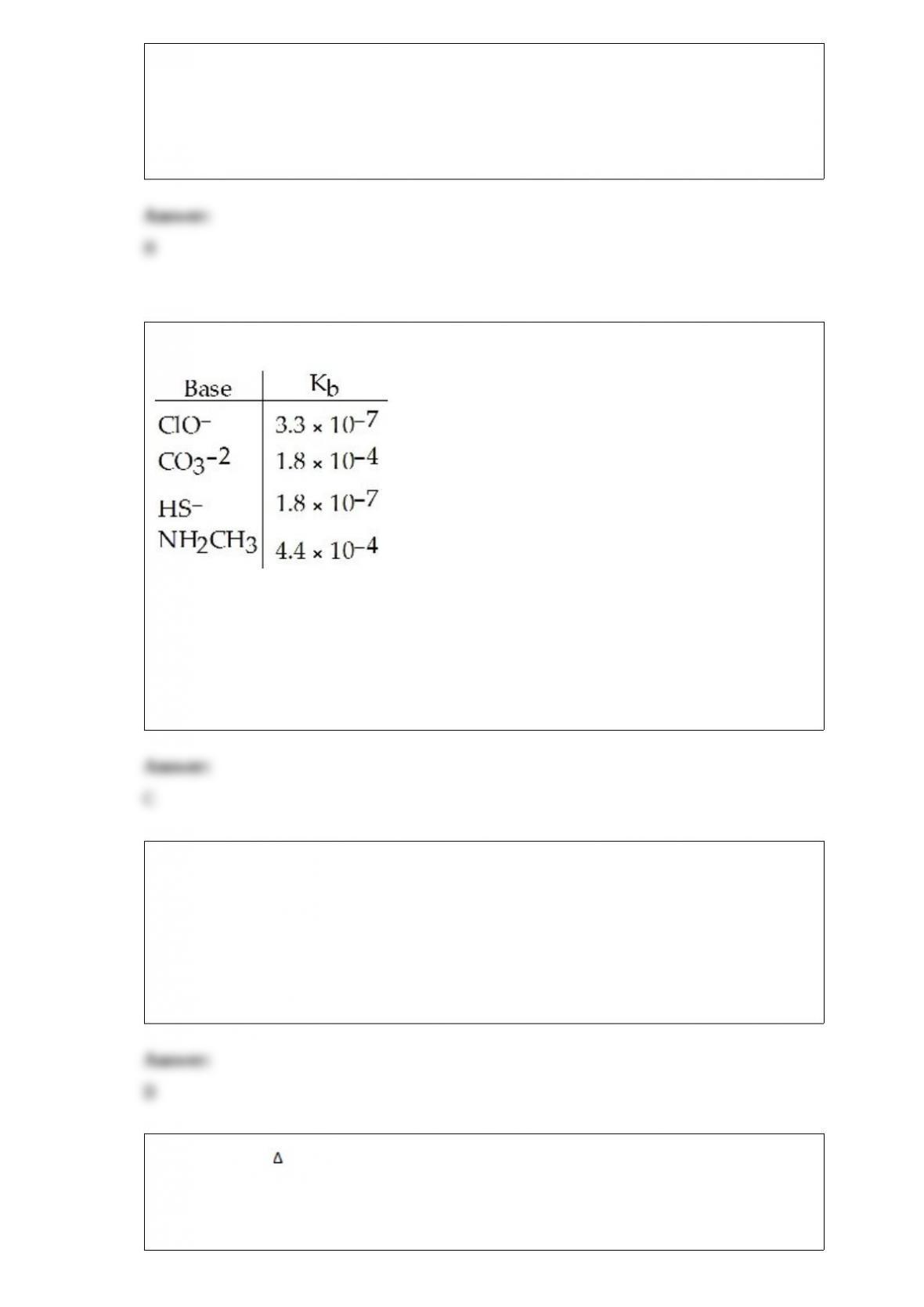
C) 0.32
D) -1.13
E) More information is needed.
7) A ________ orbital is degenerate with a 5dz
2 in a many-electron atom.
A) 5pz
B) 4dz
2
C) 5s
D) 5dxy
E) 4dzz
8) An aqueous ethanol solution (400 mL) was diluted to 4.00 L, giving a concentration
of 0.0400 M. The concentration of the original solution was ________ M.
A) 0.400
B) 0.200
C) 2.00
D) 1.60
E) 4.00
9) Eutrophication of a lake is the process of ________.
A) rapid increase in the amount of dead and decaying plant matter in the lake as a result
of excessive plant growth
B) rapid decline in the lake's pH due to acid rain
C) dissolved oxygen being depleted by an overpopulation of fish
D) stocking the lake with fish
E) restoration of the lake's dissolved oxygen supply by aerobic bacteria
10) An electron cannot have the quantum numbers n = ________, l = ________, ml =
________.
A) 6, 1, 0
B) 3, 2, 3