there are ________ mol of Br2 present in the reaction vessel.
A) 0.000
B) 0.440
C) 0.546
D) 0.136
E) 0.304
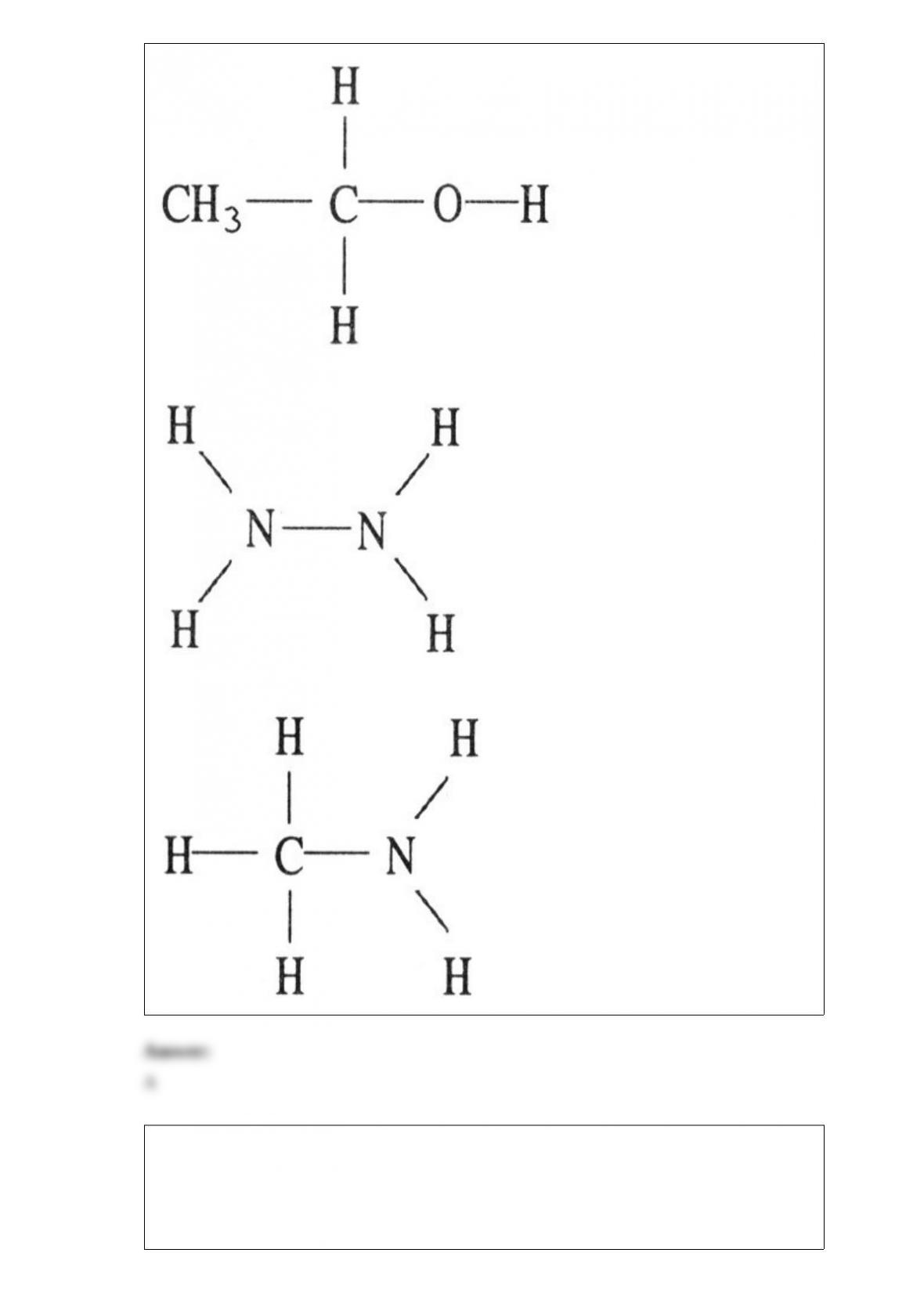
24) What types of intermolecular forces exist between NH3 and H2S?
A) dispersion forces and dipole-dipole forces
B) dispersion forces
C) dispersion forces and hydrogen bonds
D) dispersion forces, hydrogen bonds, and dipole-dipole forces
E) dispersion forces, hydrogen bonds, and ion-dipole forces
25) If an electron has a principal quantum number (n) of 7 and an angular momentum
quantum number (l) of 3, the subshell designation is ________.
A) 7f
B) 7s
C) 7p
D) 3f
E) 3d
26) Of the following transitions in the Bohr hydrogen atom, the ________ transition
results in the emission of the highest-energy photon.
A) n = 6 → n = 4
B) n = 2 → n = 7
C) n = 4 → n = 6
D) n = 1 → n = 4
E) All transitions emit photons of equivalent energy.
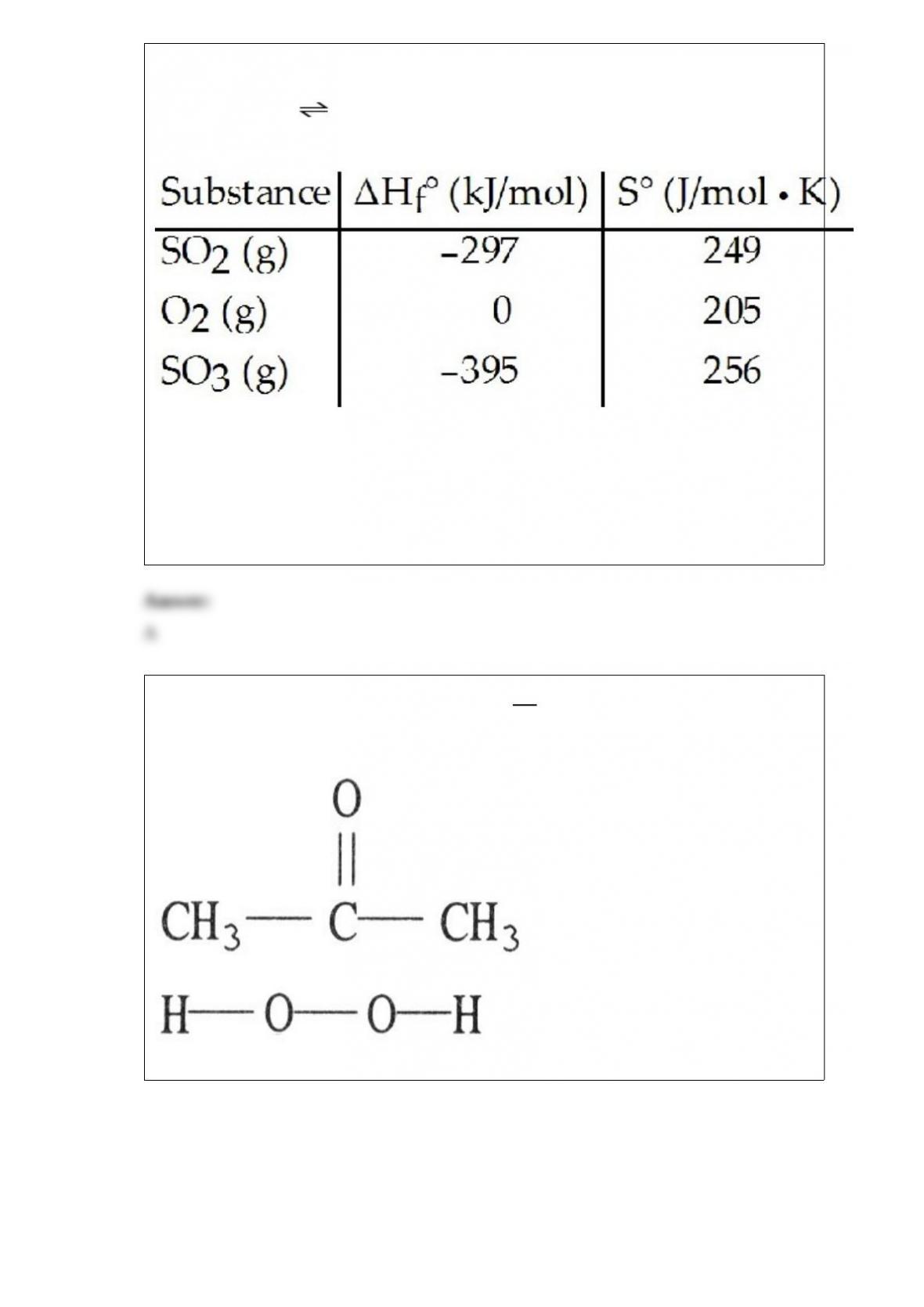
27) Given the thermodynamic data in the table below, calculate the equilibrium constant