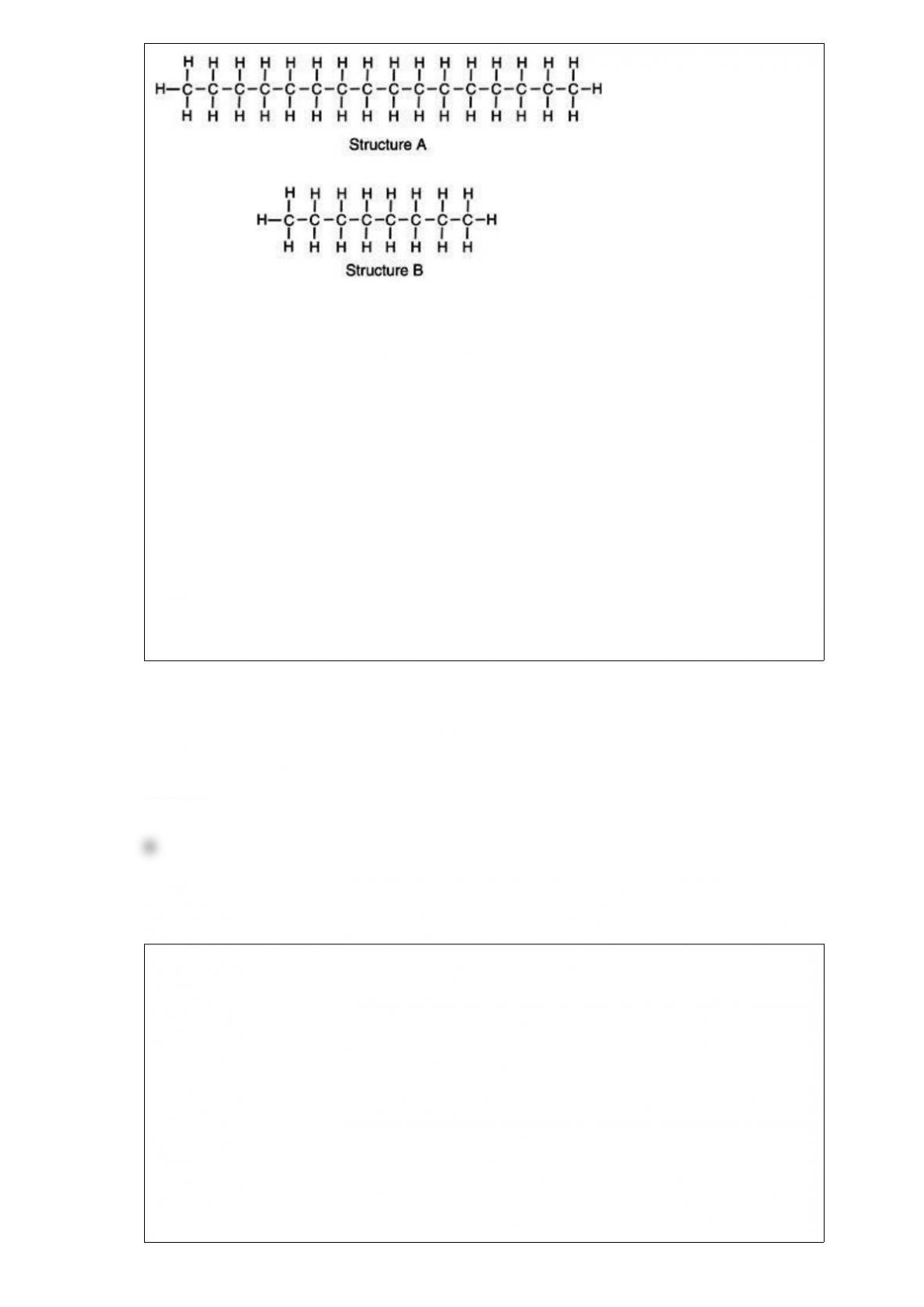
Base your reasoning not on memorization but rather upon what you know about
molecular interactions and the various physical properties of gasoline and motor oil.
A) Structure A represents the gas molecule because there are more bonds to gain energy
from, giving it a higher energy content than oil.
B) Structure A represents motor oil, illustrating a molecule with greater induced
dipole-induced dipole molecular interactions thus, the molecules are strongly attracted
to one another.
C) Structure B represents the oil molecule. Because oil molecules are smaller, they can
compact closer together, giving the appearance of a thicker solution than gasoline.
D) Structure B represents crude oil which is processed to generate longer molecules of
gasoline to prevent toxic vapors from harming consumers.
Answer:
For a falling ball the action force is the pull of Earth on the ball. The reaction force is
the
A) air resistance acting against the ball.
B) acceleration of the ball.
C) pull of the ball on Earth.
D) nonexistent.
E) none of the above