B. It must generate electricity via an acid-base reaction rather than via an
oxidation-reduction reaction.
C. The battery must be open to the outside so that it can vent any internal pressure that
builds up from gases within it.
D. The electrochemical reaction of the battery must be reversible.
Bases are substances that increase the hydroxide ion concentration in aqueous solution.
Why does ammonia (NH3), which does not contain a hydroxide group, act as a base?
A. Ammonia acts as a base only in the presence of hydroxide ion-containing
compounds.
B. Ammonia molecules remove protons from water molecules, forming hydroxide ions.
C. Ammonia molecules donate protons to water molecules, forming hydroxide ions.
D. Ammonia acts as a base only in the presence of very strong acids.
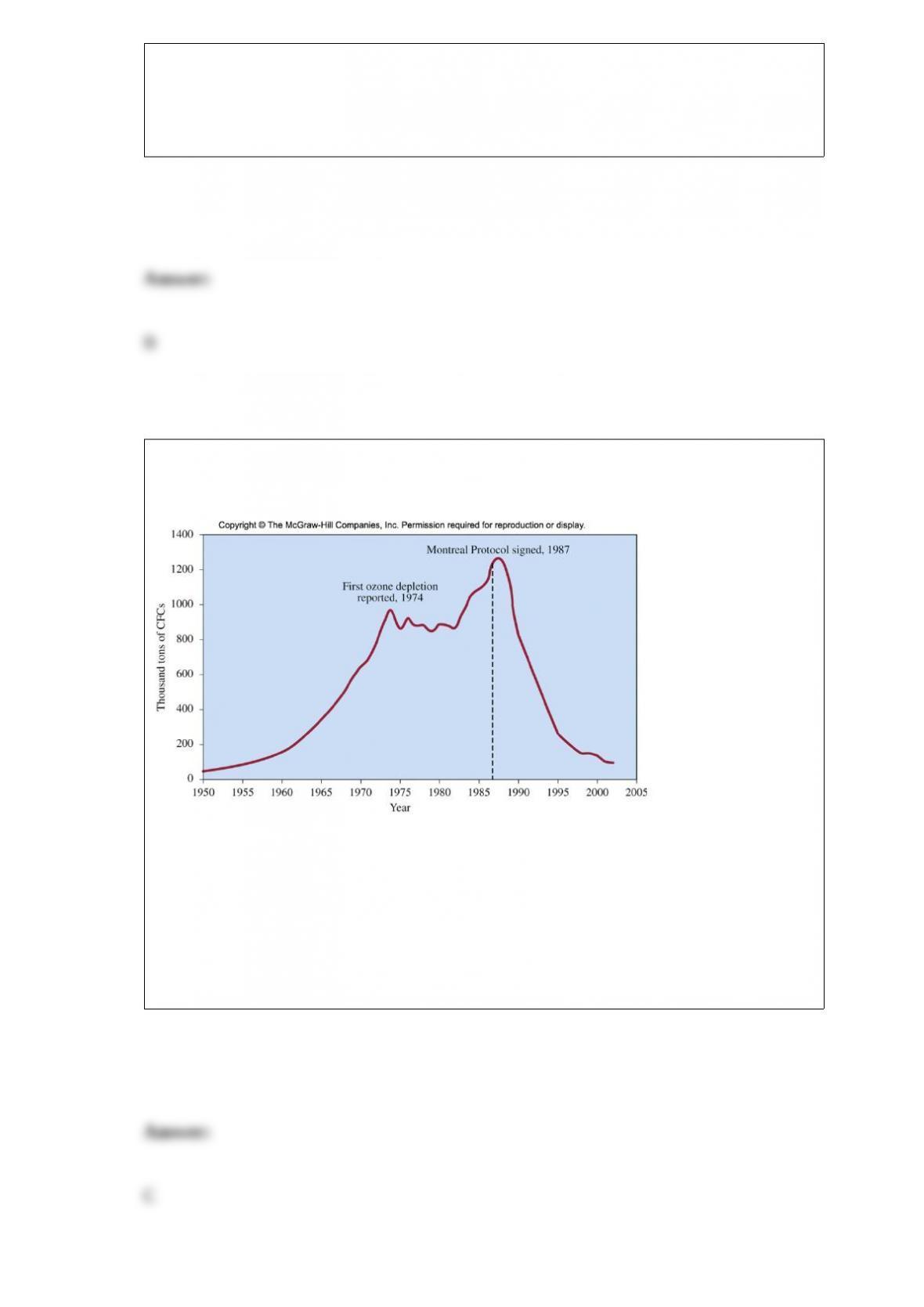
Why are HFCs inappropriate for long-term replacement of CFCs?
A. They are flammable.
B. They are very toxic.