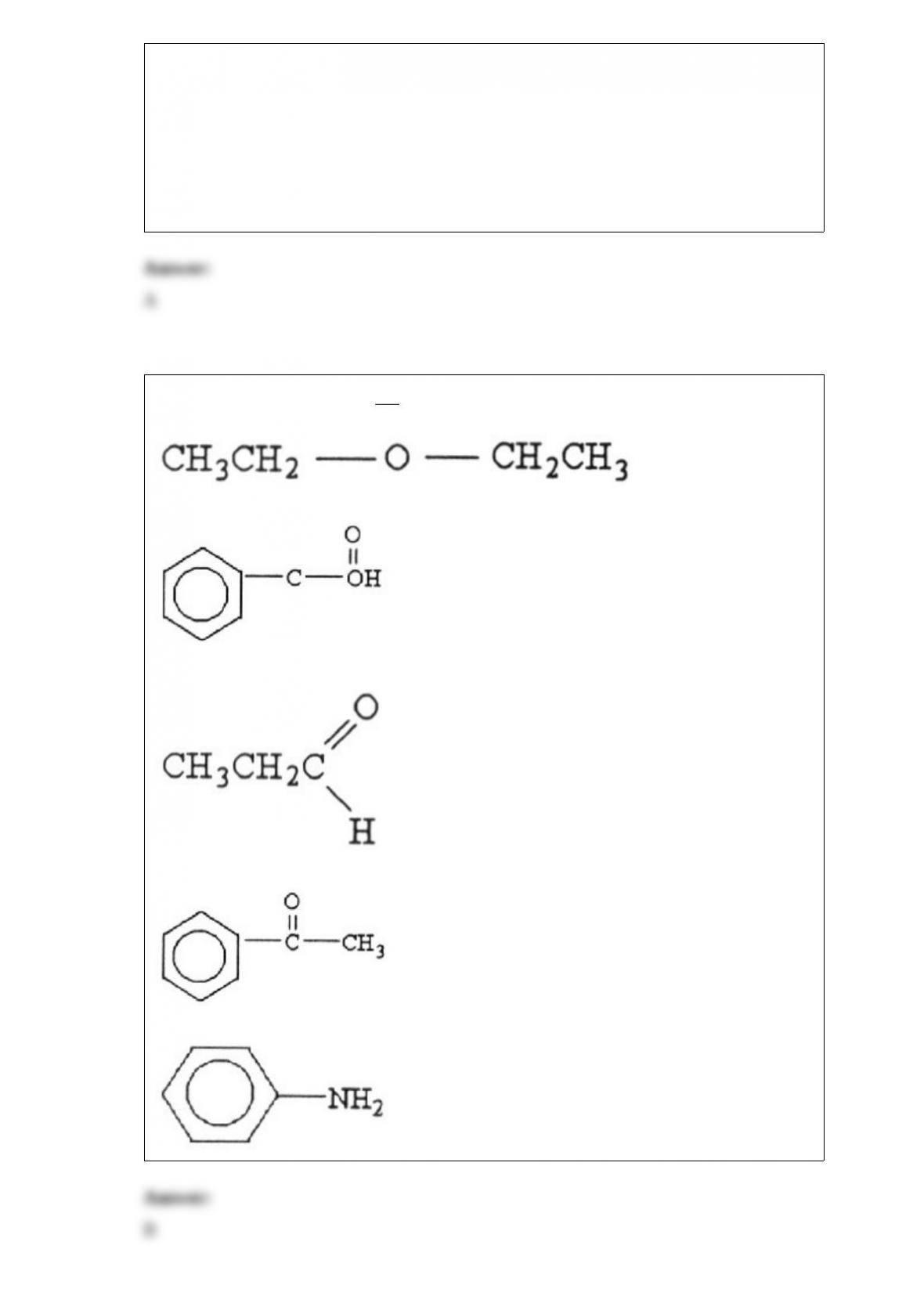
18) Of the following equilibria, only ________ will shift to the right in response to a
decrease in volume.
A) H2 (g) + Cl2 (g) 2 HCl (g)
B) 2 SO3 (g) 2 SO2 (g) + O2 (g)
C) N2 (g) + 3H2 (g) 2NH3 (g)
D) 2 Fe2O3 (s) 4 Fe (s) + 3O2 (g)
E) 2HI (g) H2 (g) + I2 (g)
19) In molecular orbital theory, the σ1s orbital is ________ and the σ1s
* orbital is
________ in the He2
+ molecule.
A) half-filled, filled
B) half-filled, half-filled
C) filled, half-filled
D) filled, empty
E) filled, filled
20) How many moles of gas are there in a 36.3 L container at 25.2 °C and 570.3 mm
Hg?
A) 1.11
B) 13.2
C) 0.863
D) 0.0110
E) 16,700
21) Consider the following reaction at equilibrium:
2SO2 (g) + O2 (g) 2SO3 (g)Ho = -99 kJ
Le Ch¢telier's principle predicts that a(n) increase in temperature will result in
________.
A) an increase in the partial pressure of O2
B) a decrease in the partial pressure of O2