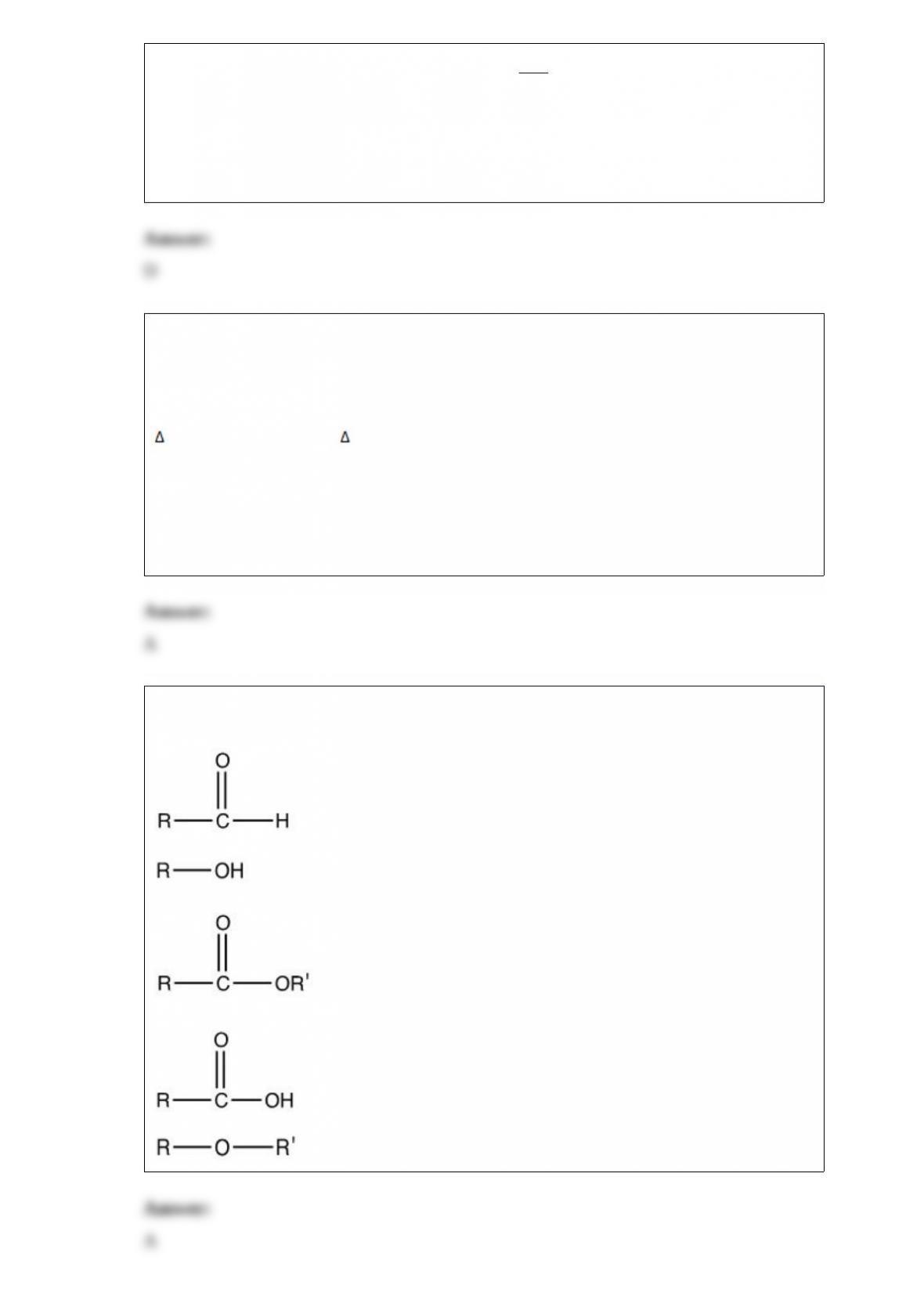
4) The temperature of a 24.3 g sample of gold increases from 23.7 °C to 31.5 °C. If the
specific heat of gold is 0.129 J/g-K, how many joules of heat are absorbed?
A) 24.5 J
B) 0.0414 J
C) -24.5 J
D) 0.293 J
E) 1.01 J
5) What is the conjugate acid of NH3?
A) NH3
B) NH2
+
C) NH3
+
D) NH4
+
E) NH4OH
6) A solution is prepared by adding 1.50 g of solid NaCl to 50.0 mL of 0.100 M CaCl2.
What is the molarity of chloride ion in the final solution? Assume that the volume of the
final solution is 50.0 mL.
A) 0.713
B) 0.613
C) 0.130
D) 0.230
E) 0.513
7) Of the reactions involved in the photodecomposition of ozone (shown below), which
are photochemical?
1. O2 (g) + h½ --> O (g) + O (g)
2. O (g) + O2(g) + M (g) --> O3(g) + M* (g)
3. O3 (g) + h½ --> O2(g) + O (g)
4. O (g) + O (g) + M (g) --> O2(g) + M* (g)
A) 2 and 4
B) 1, 2, and 4
C) 1 and 3