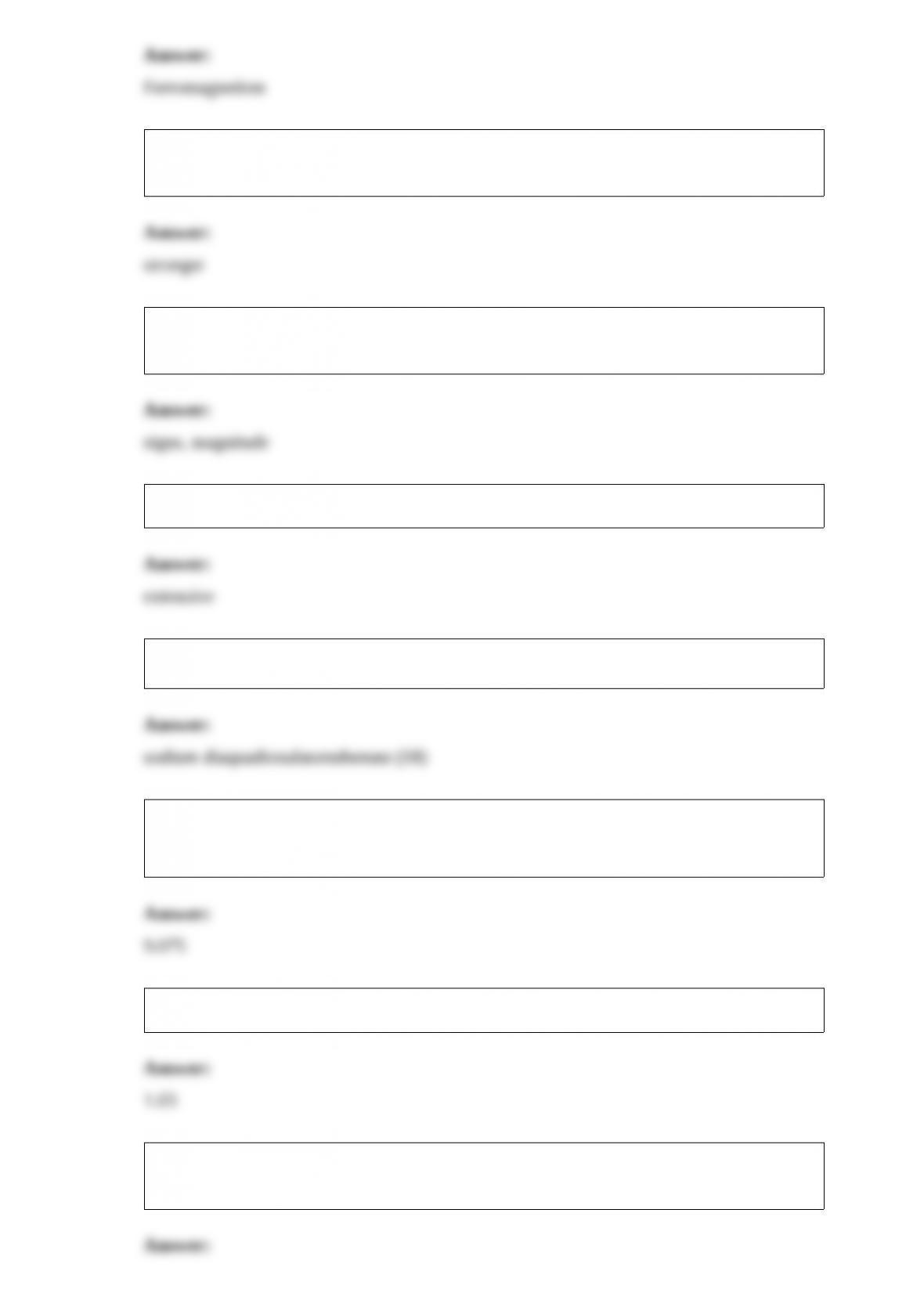
1) A reaction that is spontaneous as written ________.
A) is very rapid
B) will proceed without outside intervention
C) is also spontaneous in the reverse direction
D) has an equilibrium position that lies far to the left
E) is very slow
2) Which of the following is a valid set of four quantum numbers? (n, l, ml, ms)
A) 2, 1, 0, +1/2
B) 2, 2, 1, -1/2
C) 1, 0, 1, +1/2
D) 2, 1, +2, +1/2
E) 1, 1, 0, -1/2
3) Hydrogen is unique among the elements because ________.
1. It is not really a member of any particular group.
2. Its electron is not at all shielded from its nucleus.
3. It is the lightest element.
4. It is the only element to exist at room temperature as a diatomic gas.
5. It exhibits some chemical properties similar to those of groups 1A and 7A.
A) 1, 2, 3, 5
B) 1, 2, 3, 4, 5
C) 1, 4, 5
D) 3, 4
E) 2, 3, 4, 5
4) Which one of the following represents an acceptable set of quantum numbers for an
electron in an atom? (arranged as n, l, ml, and ms )
A) 3, 2, -2, -1/2
B) 3, 3, -4, 1/2
C) 3, 4, 6, -1/2
D) 3, 2, 0, 0
E) 3, 3, 3, -1/2