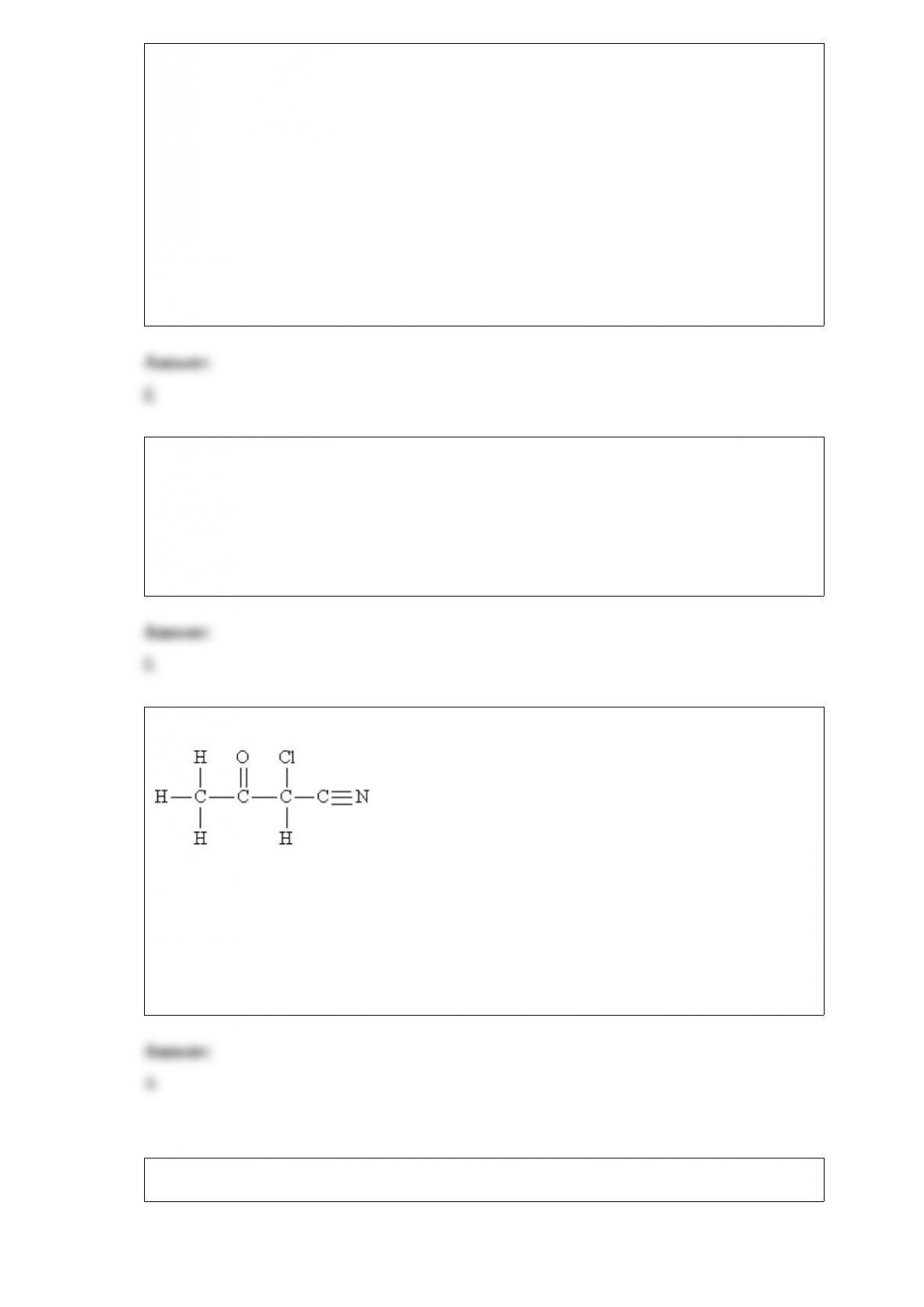
If a compound has a number of individual dipoles, then:
I.It is polar overall.
II.There is an electronegativity difference between the bonded atoms.
III.it is ionic.
IV.It doesn't have resonance.
A)II only
B)II, IV
C)I, II, IV
D)I, III
E)All of the above statements are correct.
Consider the following processes:
DH (kJ/mol)
3B --> 2C + D -125.
(1/2)A --> B 150
E + A --> D 350
Calculate DH for: B --> E + 2C
A)325 kJ/mol
B)525 kJ/mol
C)-175 kJ/mol
D)-325 kJ/mol
E)none of these
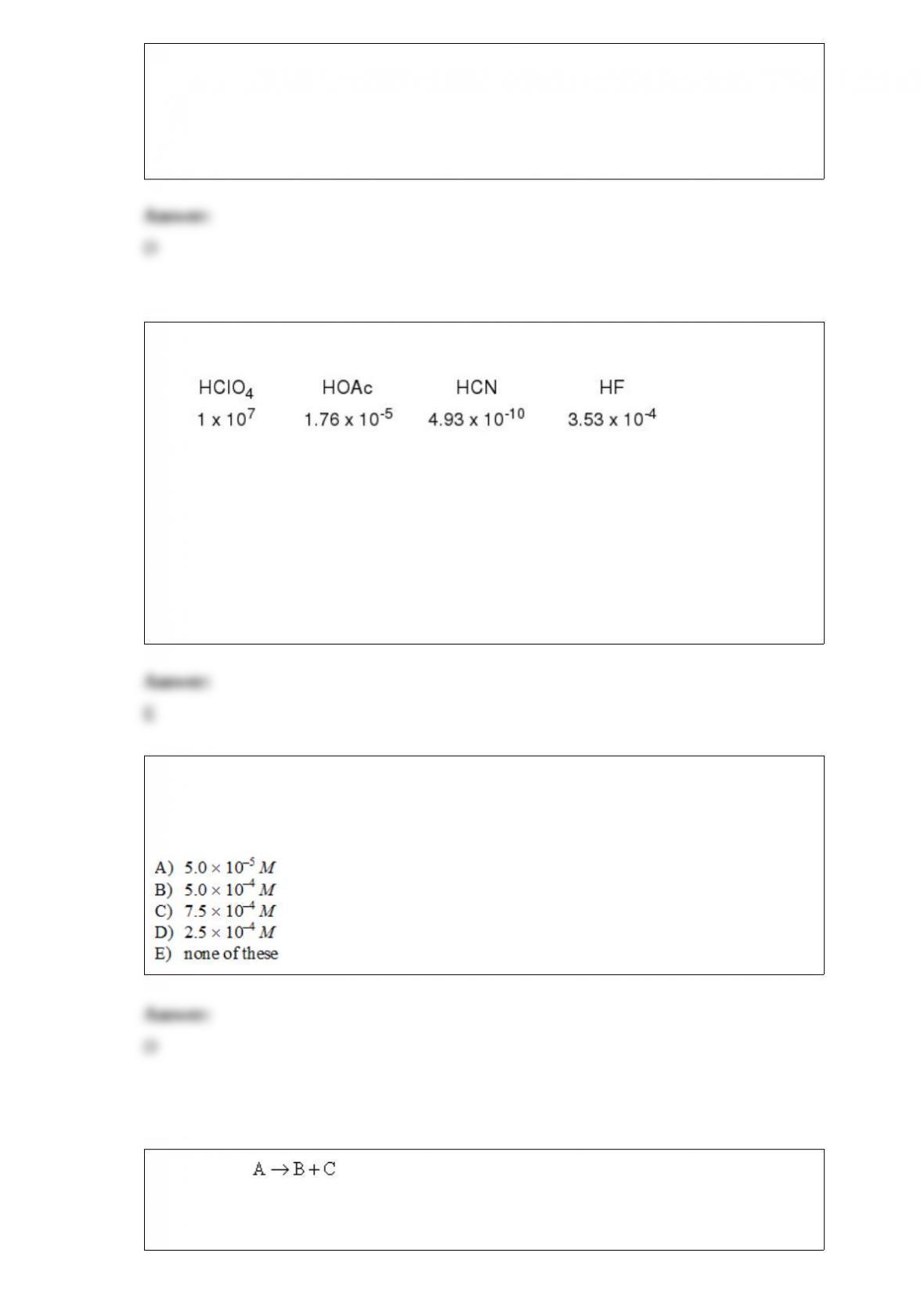
You analyze for pyridine (Kbis approximately 10-9) by dissolving 0.1000 g of complex
in 10 mL of H2O and titrating with a 0.01 M HCl solution. Which of the following
indicators should be used to detect the endpoint? (Assume that the initial concentration
of pyridine is approximately 0.01 M.)
A) bromophenol blue, pH range of color change = 3.0-4.6
B) methyl red, pH range of color change = 4.8-6.0
C) bromothymol blue, pH range of color change = 6.0-7.6
D) thymol blue, pH range of color change = 8.0 -9.6
E) alizarin yellow, pH range of color change = 10.1-12.0
Select the correct molecular structure for the given species from the choices below:
H2O