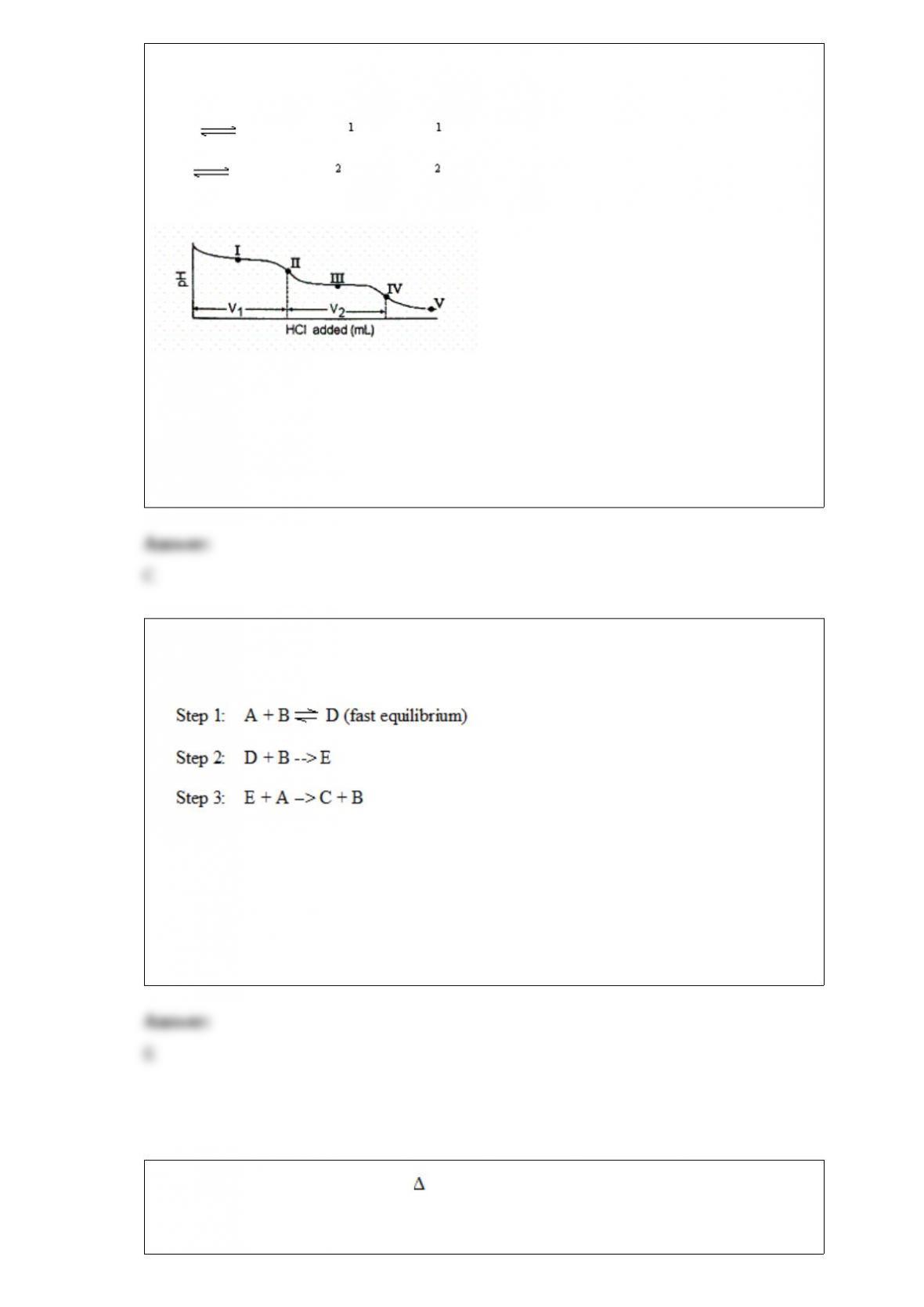
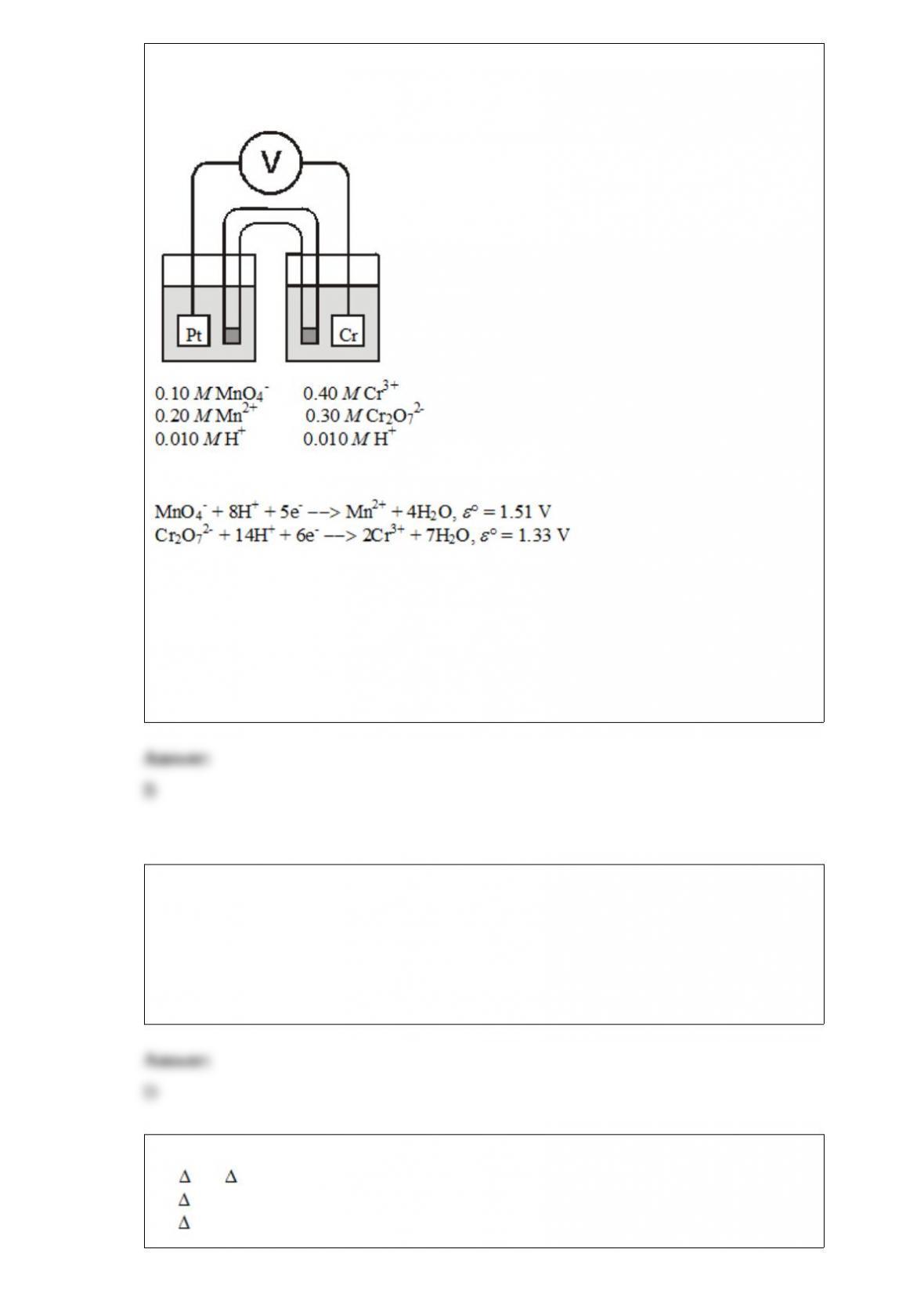
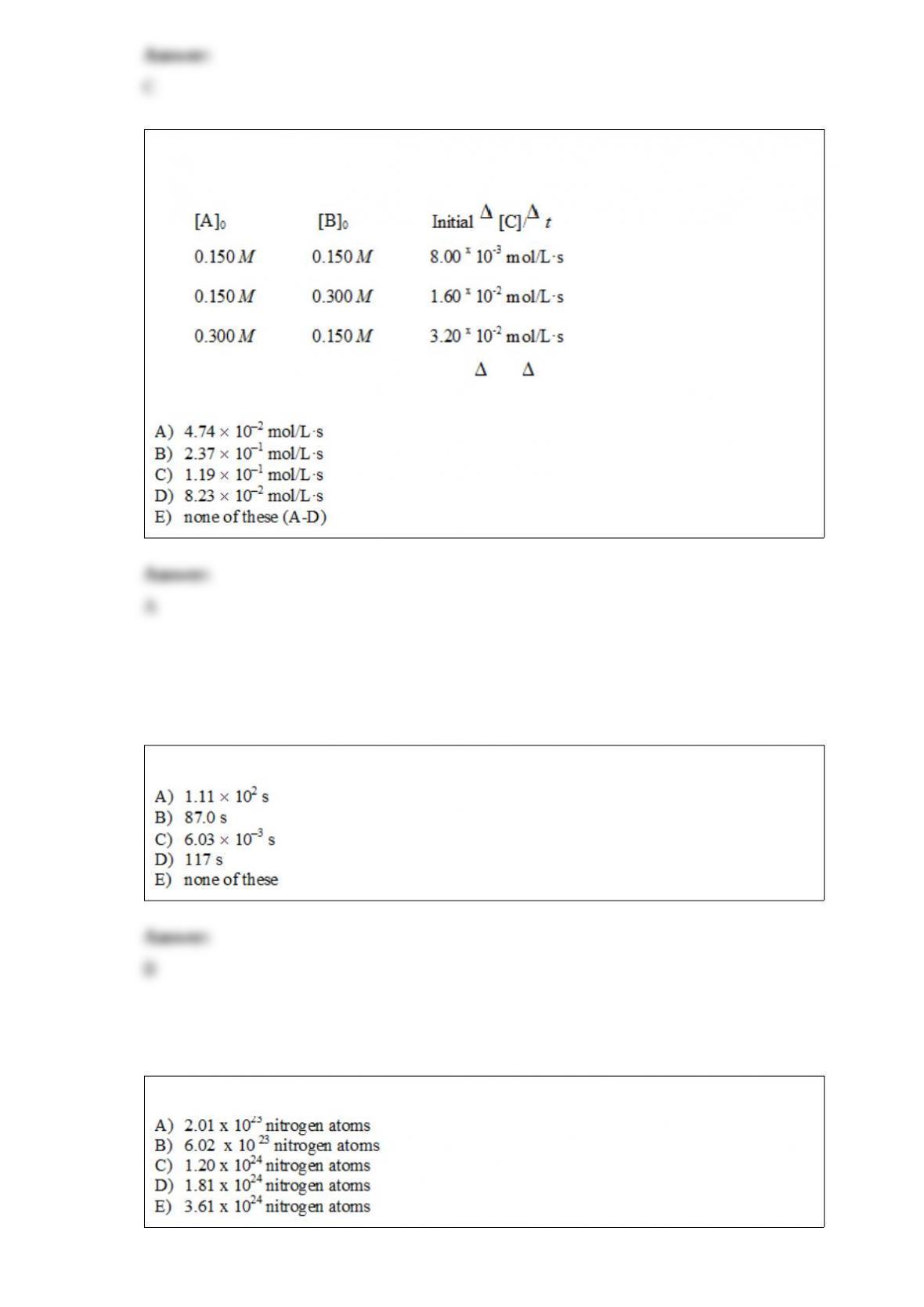
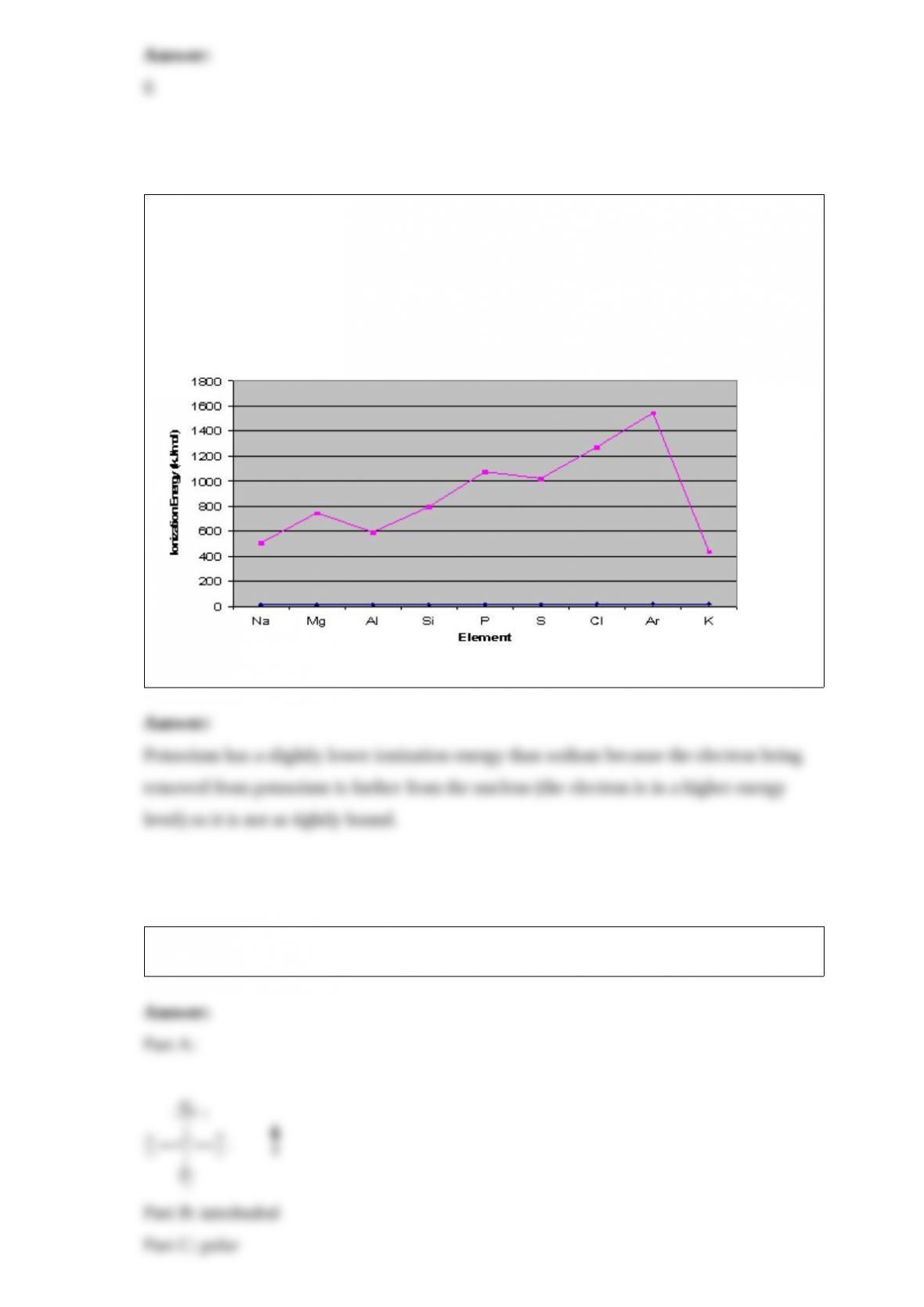
From its Lewis structure determine the following:
How many of the atoms are sp2hybridized?
A)2
B)4
C)6
D)8
E)10
Predict the sign of S° for each of the following processes:
I.2 K(s) + Cl2(g) --> 2 KCl(s)
II.CH4(g) --> C(s) + 2 H2(g)
III.CaCO3(s) --> CaO(s) + CO2(g)
A) negative, negative, positive
B) negative, negative, negative
C) positive, negative, negative
D) negative, positive, positive
E) positive, positive, positive
Which of these solutions 0.1 m NaCl, 0.15 m glucose, 0.1 m CaCl2would have
I. the highest vapor pressure
II. the highest boiling point
A)0.1 m CaCl2, 0.1 m CaCl2
B) 0.15 m glucose, 0.1 m CaCl2
C)0.1 m CaCl2, 0.15 m glucose
D)0.15 m glucose, 0.15 m glucose
E)0.1 m NaCl, 0.1 m CaCl2