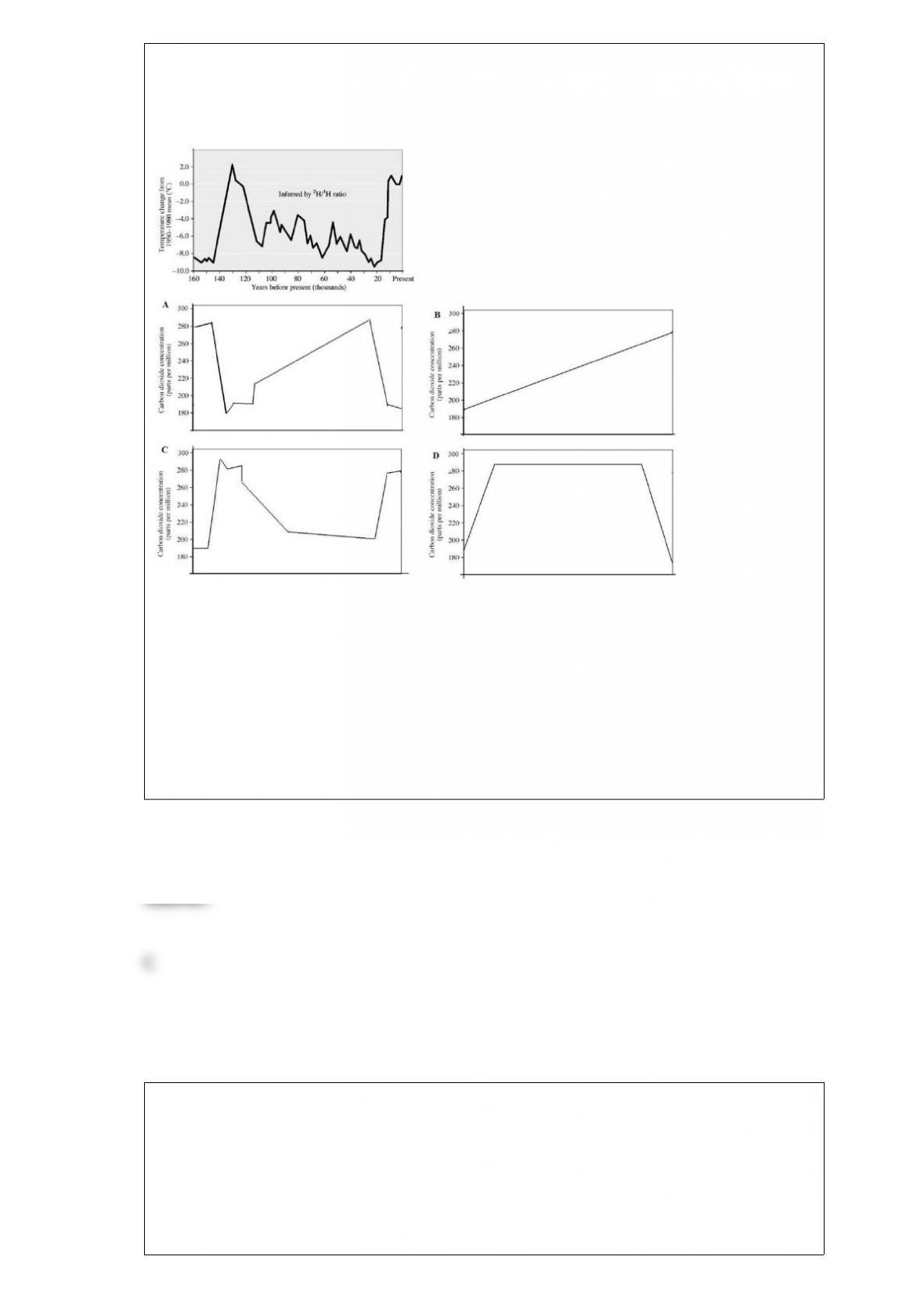
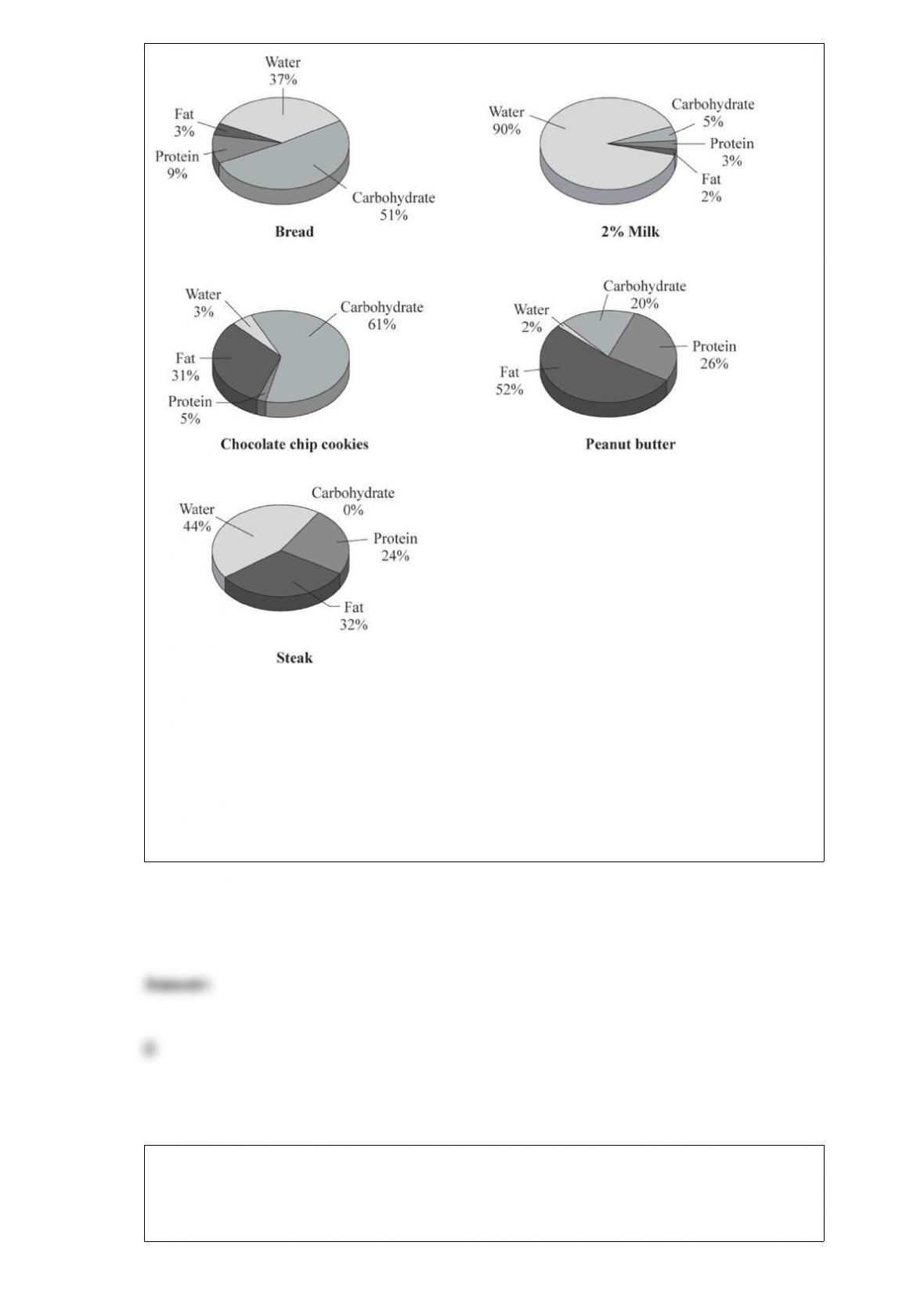
The carbon cycle refers to the
A. mechanism by which carbon dioxide causes global warming.
B. frequency of light energy that affects the rotational energy of carbon dioxide.
C. oxidation of glucose to form carbon dioxide and water.
D. movement of carbon through living organisms, the atmosphere, the sea, and the
earth.
Mark the True statement
A. In carbon containing compounds, carbon usually forms four bonds, nitrogen usually
forms three bonds, oxygen usually forms two bonds, and hydrogen only forms one
bond.
B. In carbon containing compounds, carbon usually forms four bonds, nitrogen usually
forms five bonds, oxygen usually forms six bonds, and hydrogen only forms one bond.
C. In carbon containing compounds, carbon usually forms four bonds, nitrogen and
oxygen usually form two bonds, and hydrogen only forms one bond.
D. In carbon containing compounds, carbon usually forms three bonds, nitrogen and
oxygen usually form two bonds, and hydrogen only forms one bond.