4) The curie is a measure of the ________.
A) number of disintegrations per second of a radioactive substance
B) total energy absorbed by an object e x posed to a radioactive source
C) lethal threshold for radiation e x posure
D) number of alpha particles emitted by e x actly one gram of a radioactive substance
E) None of the above is correct.
5) A typical double bond ________.
A) is stronger and shorter than a single bond
B) consists of one bond and one € bond
C) imparts rigidity to a molecule
D) consists of two shared electron pairs
E) All of the above answers are correct.
6) The lowest orbital energy is reached when the number of electrons with the same
spin is maximized. This statement describes ________.
A) Pauli Exclusion Principle
B) Planck's constant
C) deBroglie hypothesis
D) Heisenberg Uncertainty Principle
E) Hund's rule
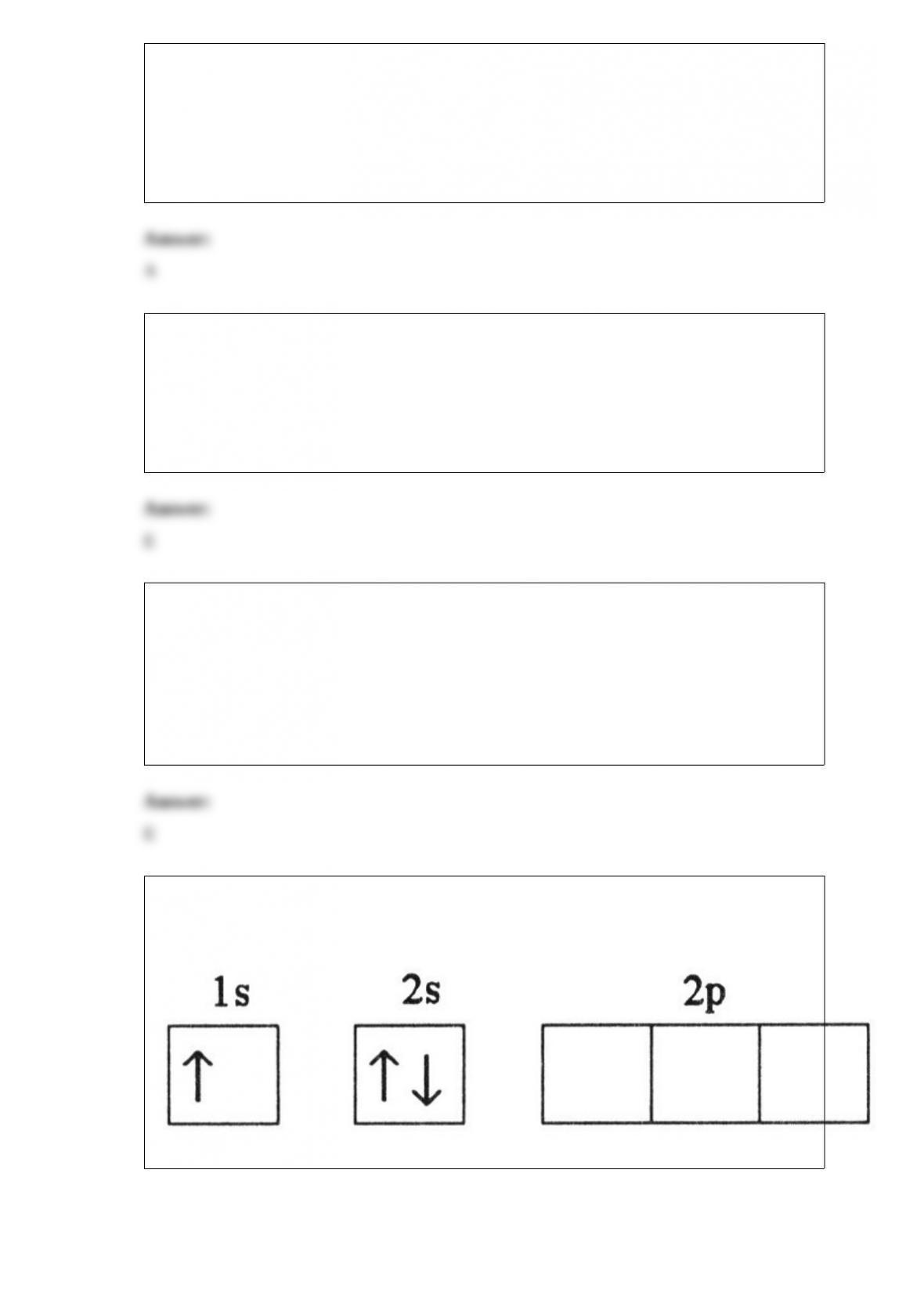
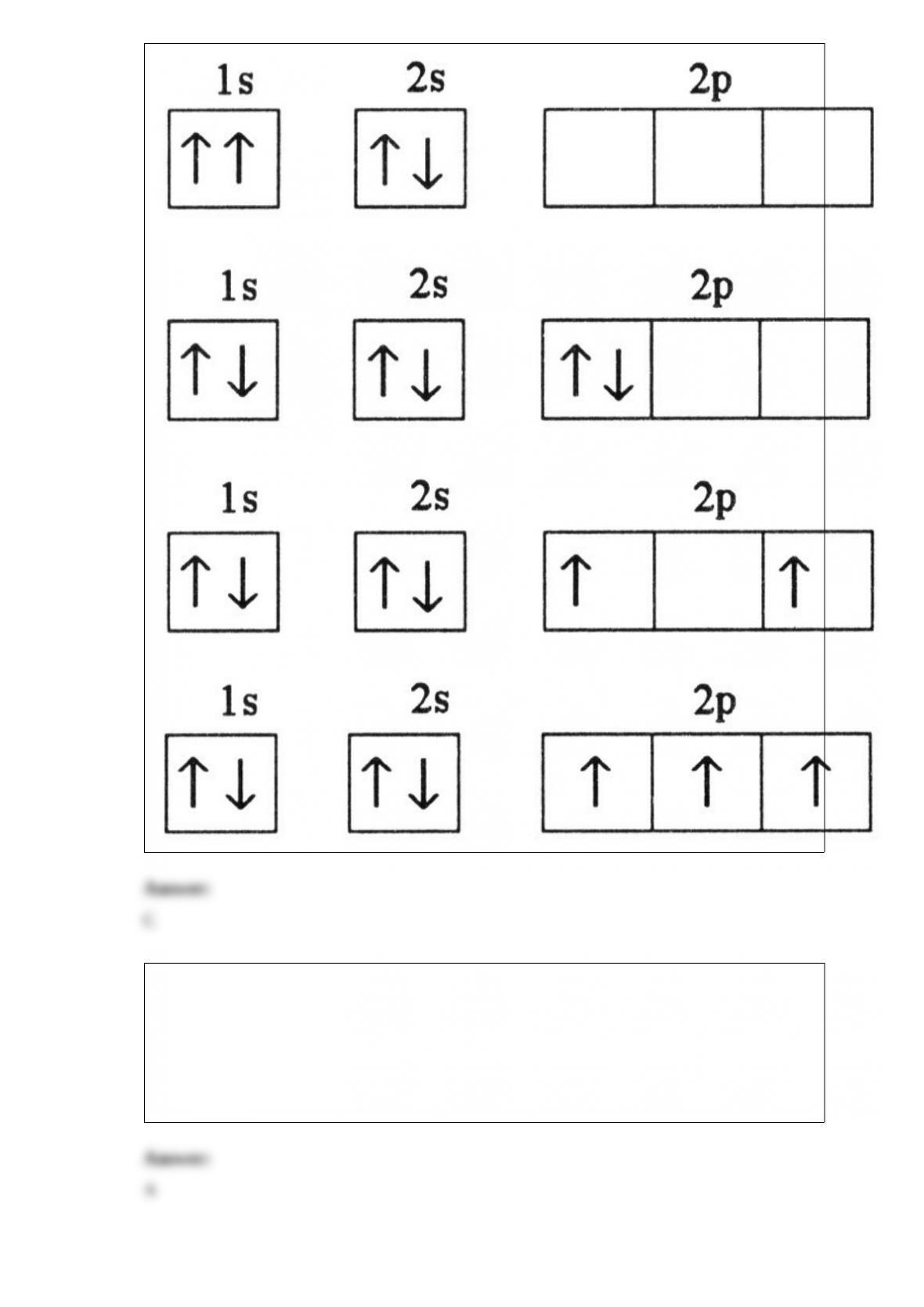
7) Which electron configuration represents a violation of Hund's rule for an atom in its
ground state?
A)
B)