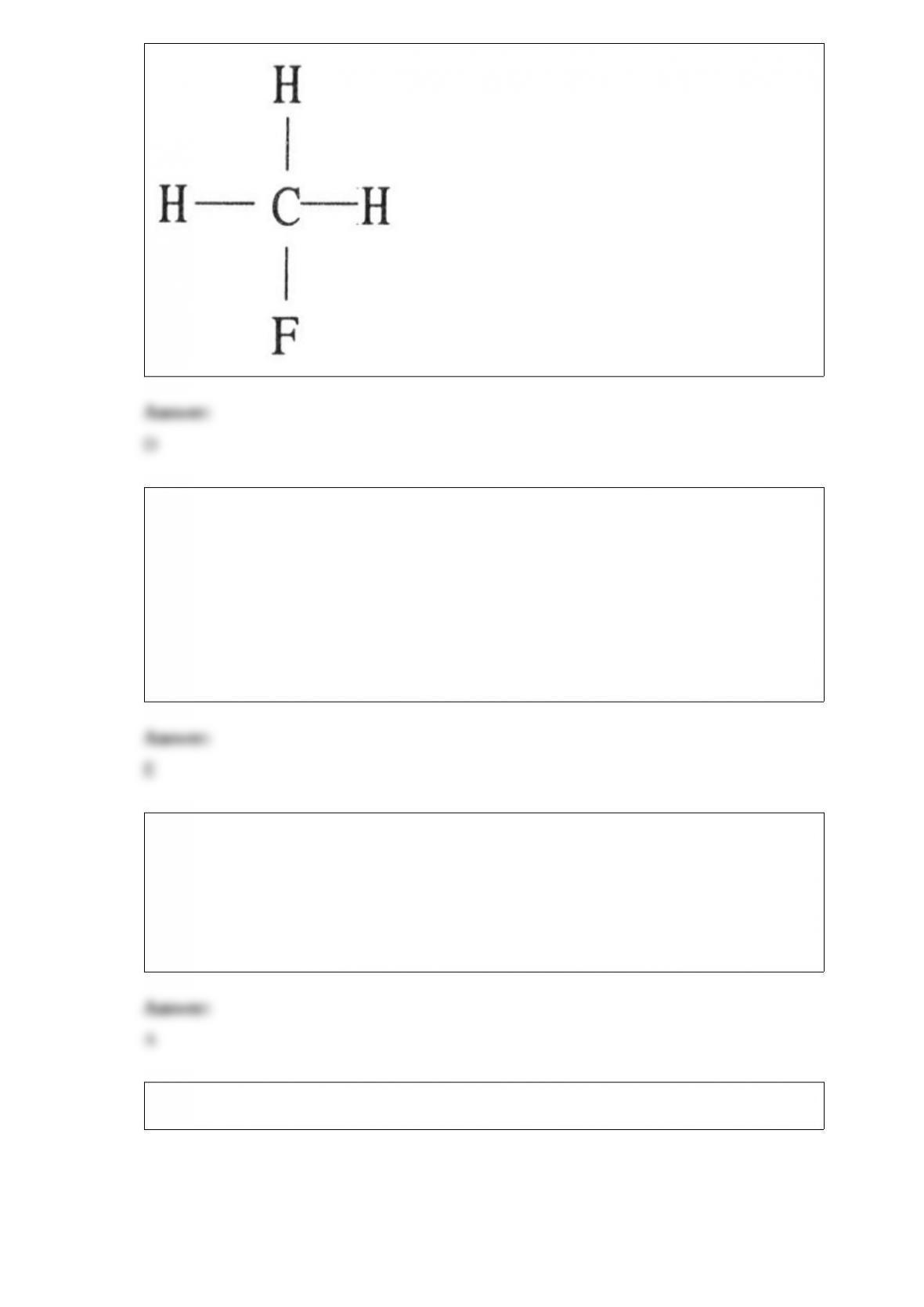
18) In the Lewis structure of HCO3
-, the formal charge on H is ________, and the
formal charge on C is ________.
A) -1, -1
B) 0, 0
C) 0, -1
D) +1, -1
E) -1, +1
19) The density of air under ordinary conditions at 25 °C is 1.19 g/L. How many
kilograms of air are in a room that measures 9.0 ft X 11.0 ft and has a 10.0 ft ceiling?
A) 2.99
B) 0.124
C) 3.34 X 104
D) 0.0644
E) 33.4
20) Of the possible bonds between carbon atoms (single, double, and triple), ________.
A) a triple bond is longer than a single bond
B) a double bond is stronger than a triple bond
C) a single bond is stronger than a triple bond
D) a double bond is longer than a triple bond
E) a single bond is stronger than a double bond