1) Which one of the following configurations depicts an excited carbon atom?
A) 1s22s22p13s1
B) 1s22s22p3
C) 1s22s22p1
D) 1s22s23s1
E) 1s22s22p2
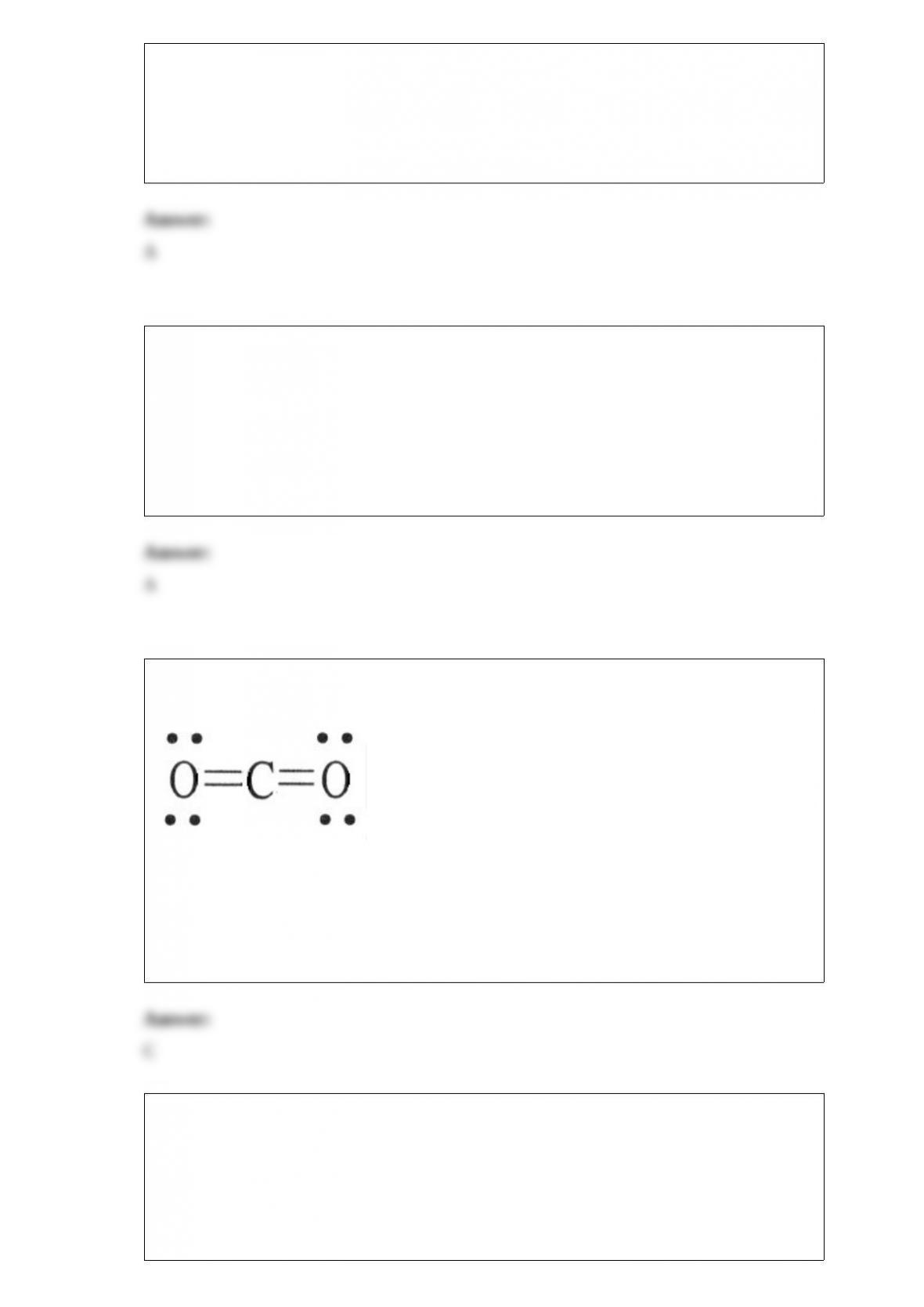
2) A carbonyl compound contains ________.
A) a carbon-oxygen double bond
B) a carbon-oxygen triple bond
C) a carbon atom with a lone pair of electrons
D) a carbon-carbon triple bond
E) a carbon-carbon double bond
3) Metals can be ________ at room temperature.
A) liquid only
B) solid only
C) solid or liquid
D) solid, liquid, or gas
E) liquid or gas
4) The oxidation numbers of sulfur in the sulfate ion, sulfite ion, sulfur trioxide, and
hydrogen sulfide are ________, ________, ________, and ________, respectively.
A) +4, -2, +4, +6
B) +6, +2, +4, +6
C) +6, +4, +6, -2
D) +4, +6, +4, -2
E) -2, +6, -2, 0
5) The balanced equation for the decomposition of sodium azide is ________.
A) 2NaN3 (s) → 2Na (s) + 3N2 (g)