B) a decrease in the total pressure (T constant)
C) addition of some N2 to the reaction vessel (V and T constant)
D) a decrease in the total volume of the reaction vessel (T constant)
E) an increase in total pressure by the addition of helium gas (V and T constant)
9) A reaction vessel is charged with hydrogen iodide, which partially decomposes to
molecular hydrogen and iodine:
2HI (g) H2(g) + I2(g)
When the system comes to equilibrium at 425 oC, PHI = 0.708 atm, and PH2 = PI2 =
0.0960 atm. The value of Kp at this temperature is ________.
A) 6.80 x 10-2
B) 1.30 x 10-2
C) 54.3
D) 1.84 x 10-2
E) Kp cannot be calculated for this gas reaction when the volume of the reaction vessel
is not given.
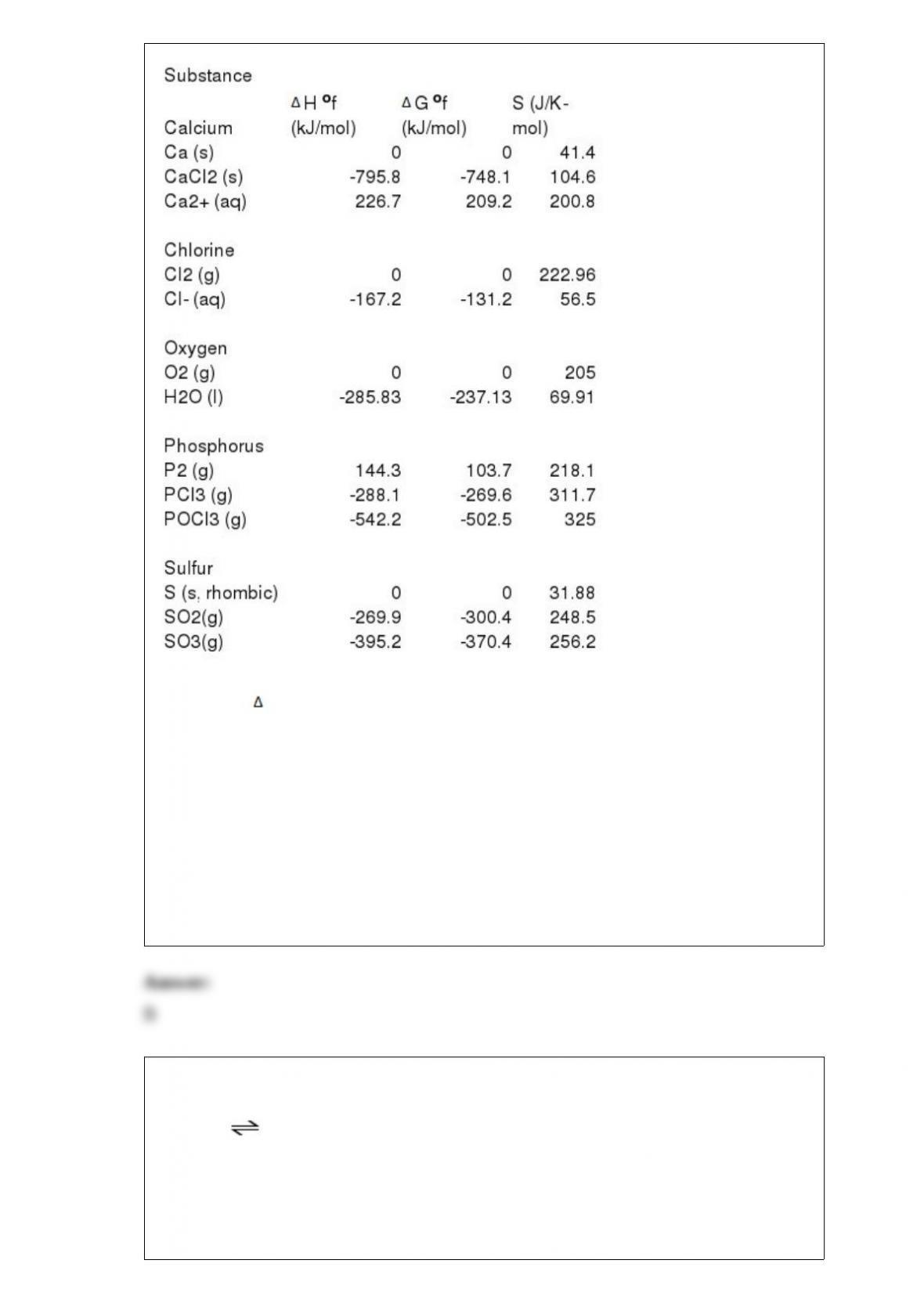
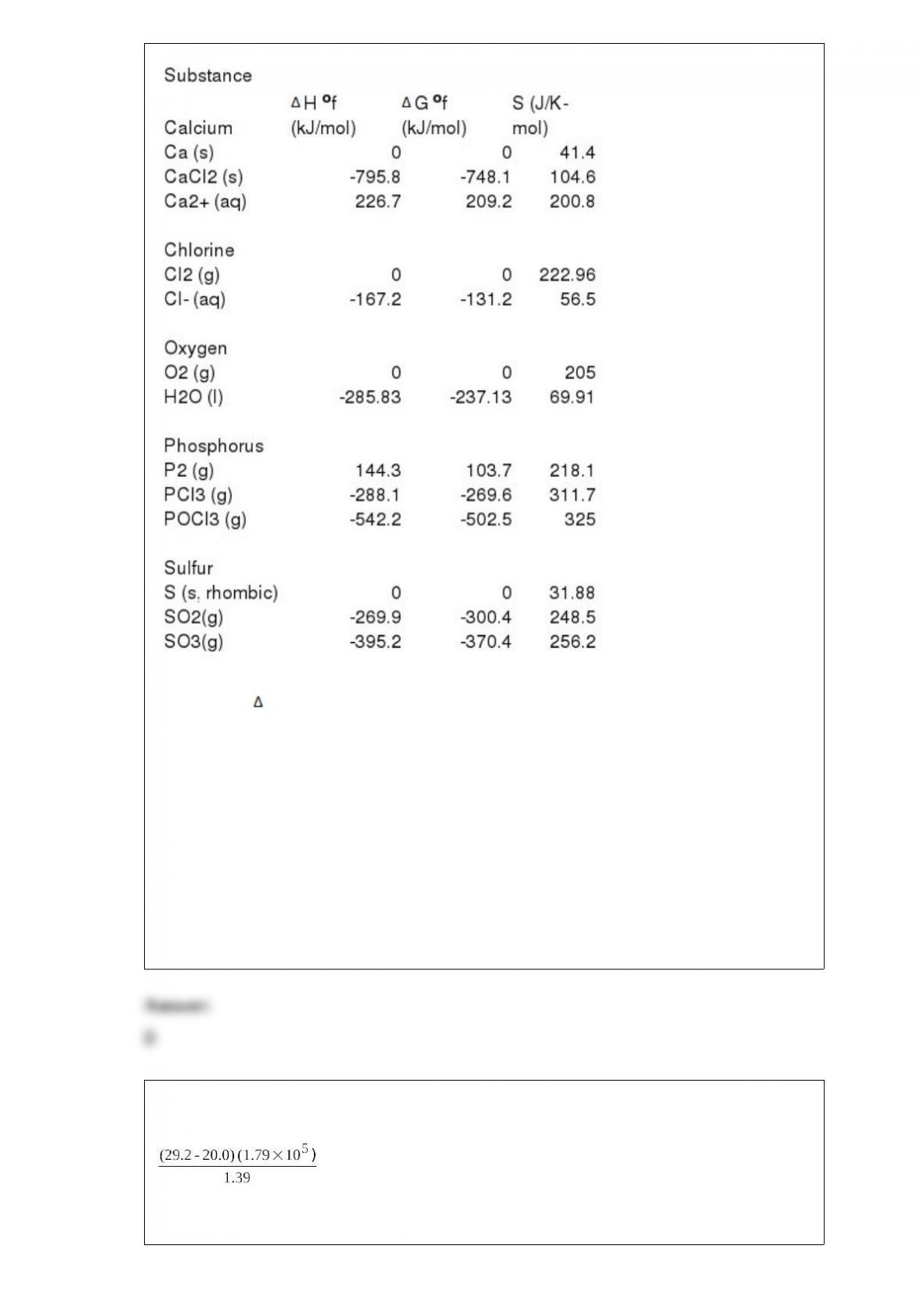
10) Phosphorous and chlorine gases combine to produce phosphorous trichloride:
P2 (g) + 3Cl2 (g) --> 2PCl3 (g)
G o at 298 K for this reaction is -642.9 kJ/mol. The value of G at 298 K for a
reaction mixture that consists of 1.9 atm P2, 1.6 atm Cl2, and 0.65 atm PCl3 is
________.
A) -34.9
B) -4.46 x 103
C) -7.86 x 103
D) -714.1
E) -650.1
11) What is the most common geometry found in four-coordinate complexes?
A) square planar