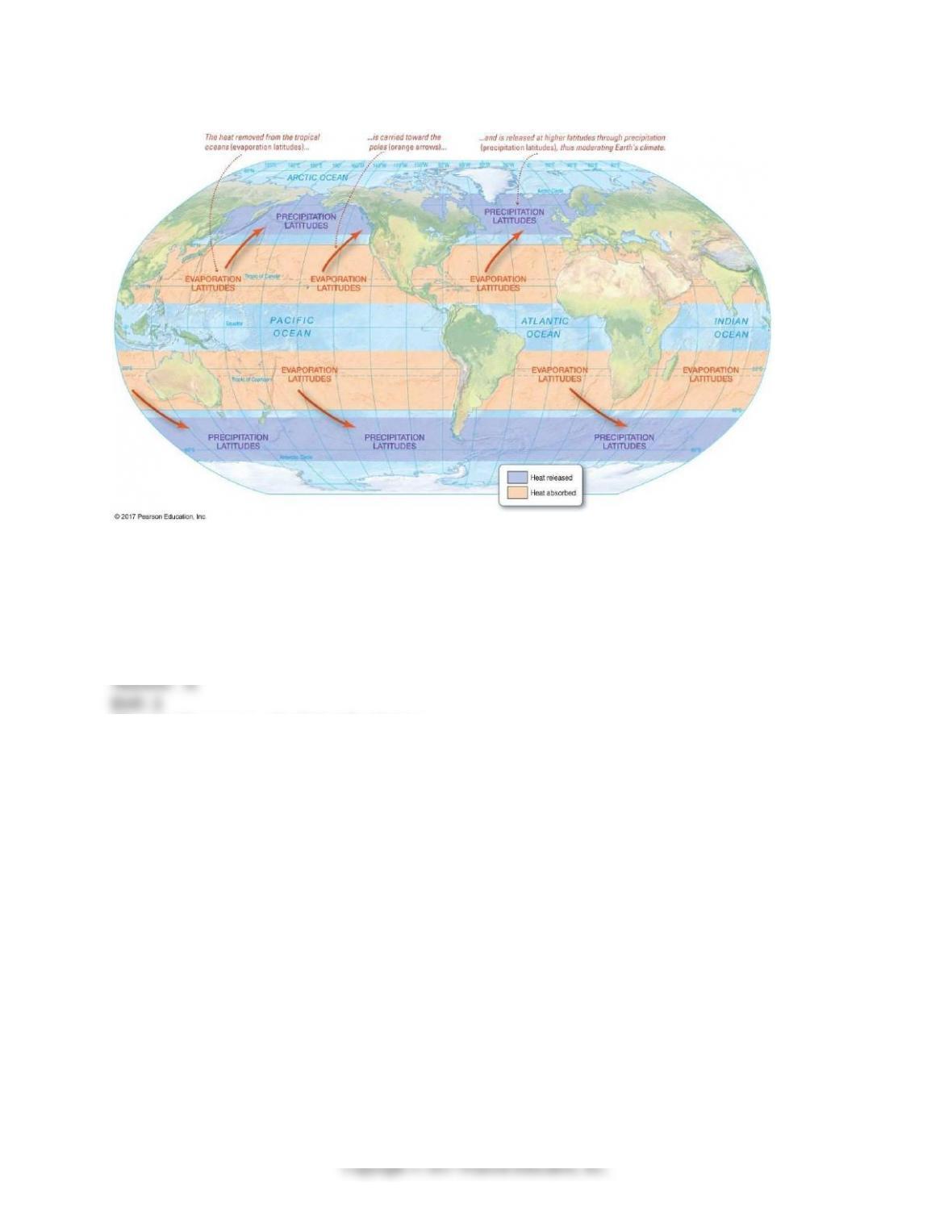
7) Explain how seawater salinity is affected by surface processes (such as precipitation and
evaporation, for example).
Bloom's Taxonomy: Remembering/Understanding
Section: 5.4 Why Does Seawater Salinity Vary?
Essent'l Concept: 5.4 Explain why seawater salinity varies
8) How does the ocean's salinity vary with depth?
Bloom's Taxonomy: Remembering/Understanding
Section: 5.6 How Does Seawater Salinity Vary at the Surface and with Depth?
Essent'l Concept: 5.6 Specify how seawater salinity varies at the surface and with depth
9) Discuss the relationship between seawater density and water temperature.
Bloom's Taxonomy: Remembering/Understanding
Section: 5.7 How Does Seawater Density Vary with Depth?
Essent'l Concept: 5.7 Specify how seawater density varies with depth
10) Describe the various methods used to desalinate seawater.
Answer: Electrolysis Method: A current is run through positive and negative electrodes in
freshwater separated by semipermeable membranes from seawater. Distillation Method:
Saltwater is boiled and the water vapor is passed through a cooling condenser, where it
condenses as freshwater. Freeze Separation Method: Seawater is frozen and thawed multiple
times, with the salts washed from the ice between each thawing. Reverse Osmosis Method:
Water on the salty side of a semipermeable membrane is pushed under high pressure through the
membrane to the freshwater side.
Diff: 1
Bloom's Taxonomy: Remembering/Understanding
Section: 5.8 What Methods Are Used to Desalinate Seawater?
Essent'l Concept: 5.8 Compare the methods used to desalinate seawater
32
Copyright © 2017 Pearson Education, Inc.