Ramon Tienda
TA: Thinh Luong
Chem 008L 08
13 March 2017 Extraction
Introduction:
The purpose of the lab was to understand the technique of extraction. Extraction is a way
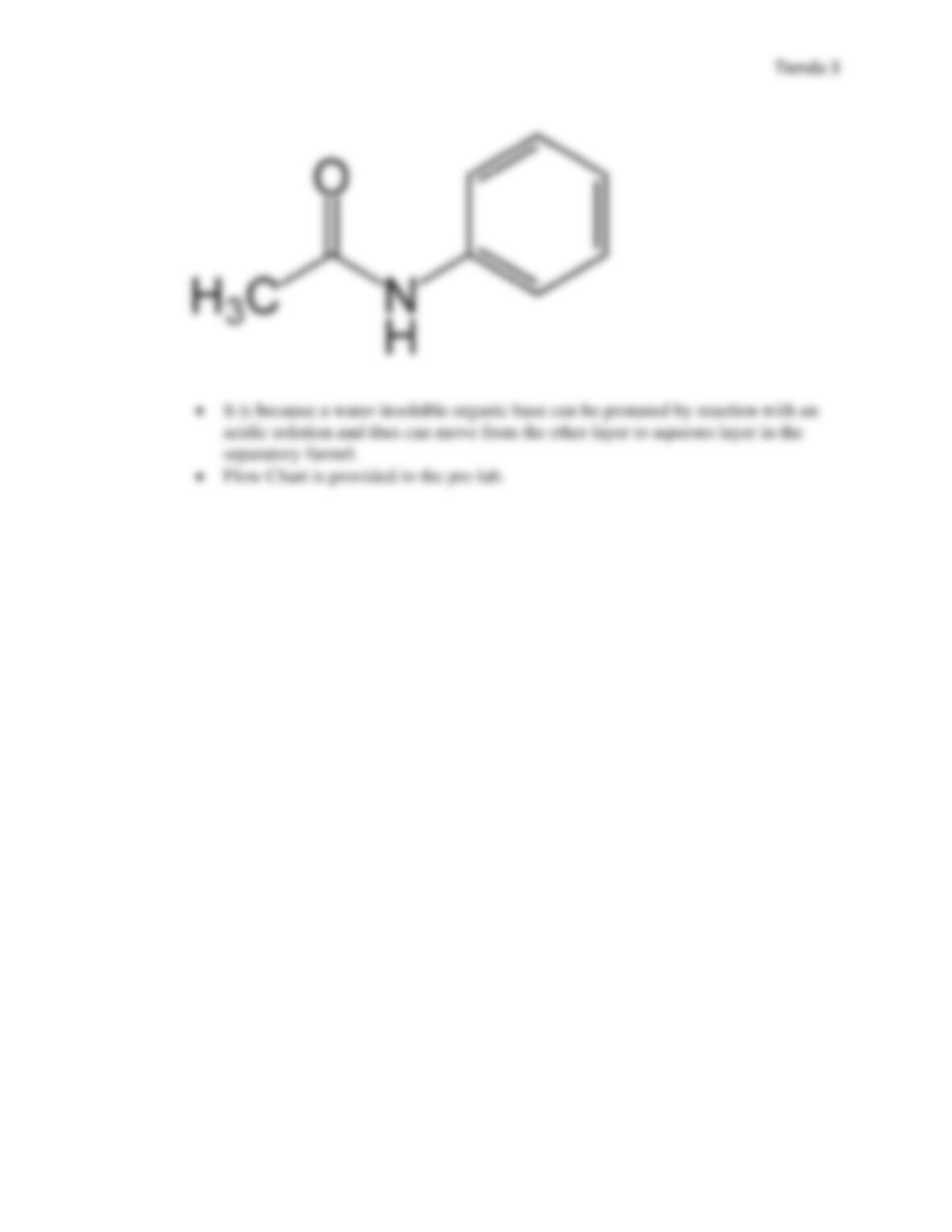
of separating water soluble chemicals from organic compounds. A mixture of urea, acetanilide,
and aspirin (acetylsalicylic acid) was to be separated using the lab technique of extraction. A
separatory funnel was used to facilitate a liquid-liquid extraction. Urea had a percent recovered
of 74.2% with a melting point range of 131.0oC – 134.1oC. Acetanilide did not have any
recovery due to human error which will be explained later in the procedure. Aspirin had a
percent recovery of 112.8% with a melting range of 135.4oC – 136.8oC.
Procedure:
A mixture of urea, acetanilide, and aspirin was measured out and weighed 1.501 g. 25
mL of t-butyl methyl ether was added to the mixture and mixed together. The mixture was
vacuumed to collect the undissolved solid which was the urea. Residue stayed on the side of the
flask, even after multiple attempts of rinsing with t-butyl methyl ether, which may have caused
the reason for a lower percent recovery than needed. A 125 mL separatory flask was assembled,
the filtrate was then rinsed with 5 mL t-butyl methyl ether, and then was added to the funnel. The
t-butyl methyl ether was extracted with 13 mL of 1 M sodium hydroxide, and placed in a beaker.
The process was done again, but this time the beaker contained combined aqueous layers. This is