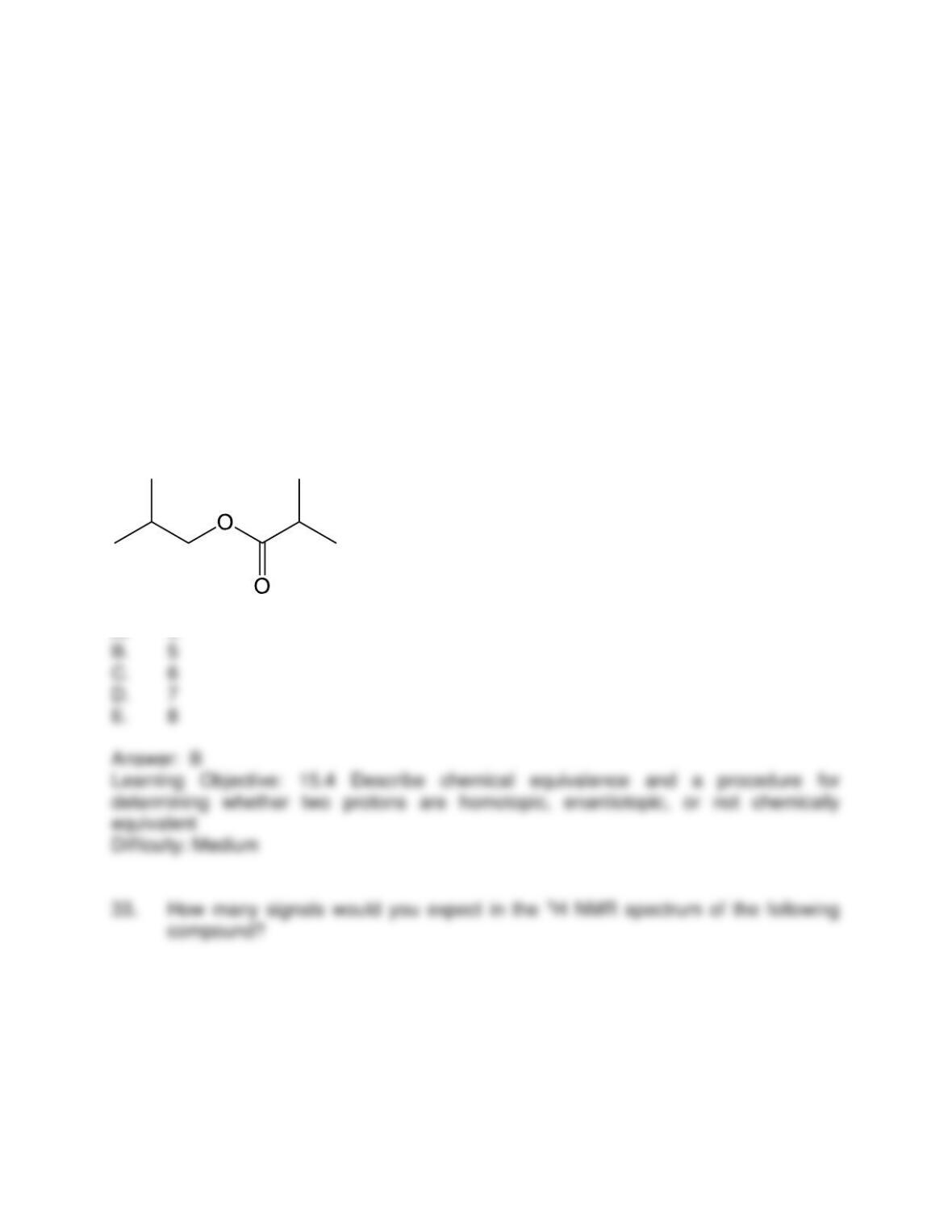
B. it indicates the electronic environment of neighboring protons
C. it indicates the number of different protons
D. it indicates the electronic environment of absorbing protons
E. it indicates the relative number of protons in the signal
Answer: E
Learning Objective: 15.3 State the significance of the number of signals, along with their
locations, areas, and shapes, in am NMR spectrum
Difficulty: Easy
15. Which of the following is true about the shape (multiplicity) of the signal in a 1H
NMR spectrum?
A. it indicates the number of neighboring protons
B. it indicates the electronic environment of neighboring protons
C. it indicates the number of different protons
D. it indicates the electronic environment of absorbing protons
E. it indicates the number of protons in the signal
Answer: A
Learning Objective: 15.3 State the significance of the number of signals, along with their
locations, areas, and shapes, in am NMR spectrum
Difficulty: Easy
16. In an NMR spectrometer, the receiver coil records a complex signal, called a
________________, which is converted into a spectrum via a mathematical
technique called a ____________________.
Answer: free induction decay (FID), Fourier transform (FT)
Learning Objective: 15.3 State the significance of the number of signals, along with their
locations, areas, and shapes, in am NMR spectrum
Difficulty: Easy
17. Which of the following type(s) of protons are chemically non-equivalent?
A. homotopic
B. enantiotopic
C. diastereotopic
D. A & B
E. B & C
Answer: C