Brittany Hoang
Using An Alu Insertion Polymorphism to Study Human
Populations
Introduction
Every individual has different sequences in many chromosome regions. Such sequences are
polymorphic, and are used to study human evolution, as well as disease and identity testing. This
experiment will examine polymorphism in human genome that is caused by the insertion of an
Alu transposon. Alu is a member of the family of short interspersed elements (SINEs) and is
approximately 300 nucleotides in length. Although Alu is sometimes called a jumping gene, it is
not a proper gene because it does not produce a protein product. Alu transposons are found only
in primate genomes and have accumulated in large numbers since primates diverged from other
mammals. Human chromosomes contain more than one million Alu copies. Each Alu is a unique
transposition event that occurred once in primate history. Over the years there has been a “fix”,
which means that all primates have inherited Alus on each of the paired chromosomes. However,
Alus have been inserted in our genome since humans branched from other primates. Some aren’t
“fixed” which means the insertion may be present or absent on each of the paired chromosomes,
creating two possible alleles.
Purpose
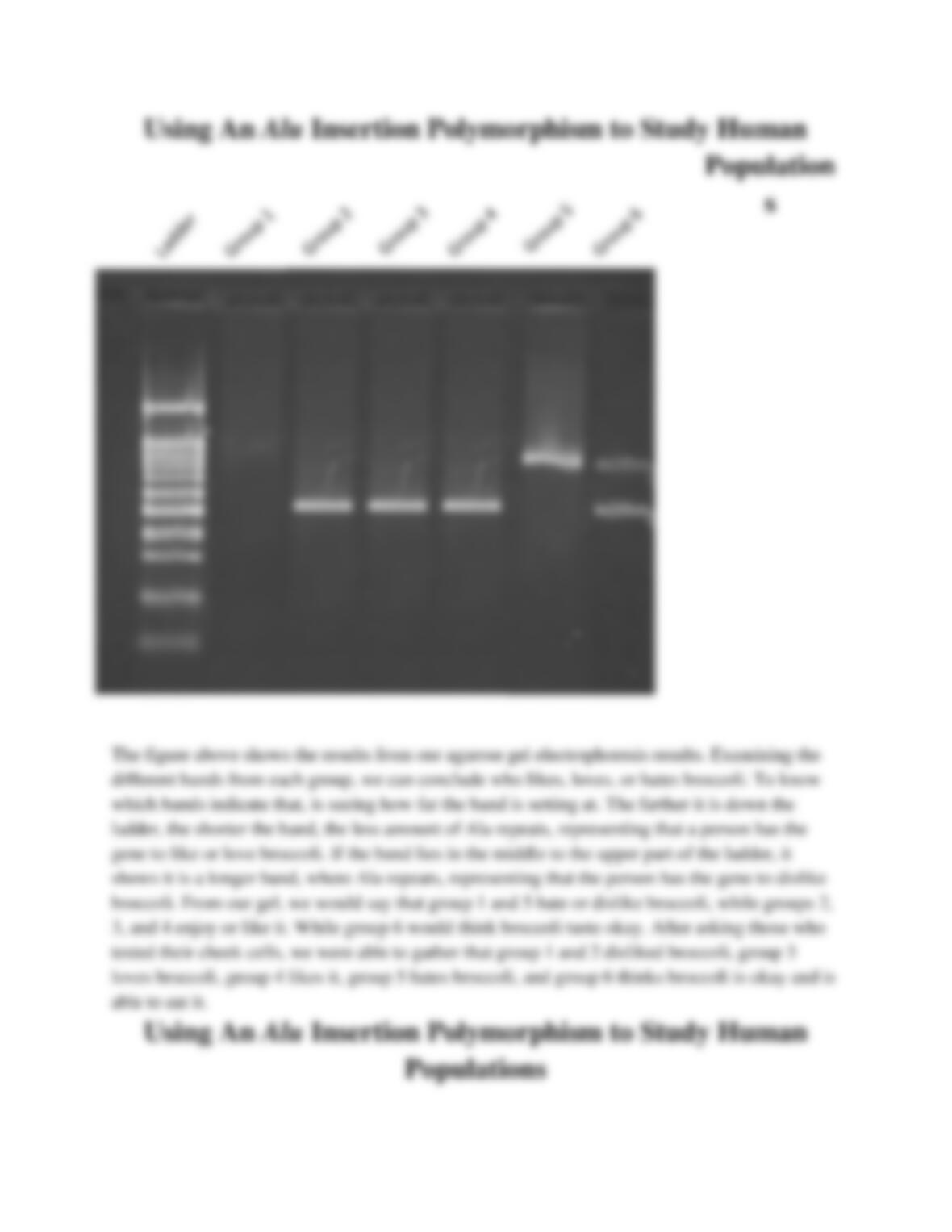
We are trying to examine a human Alu dimorphism at the PV92 locus using human cheeks cells.
From this, we will be able to distinguish if and why some people enjoy, like, or hate broccoli.
Materials
The materials included in this experiment are permanent marker, paper cup, micropipette and
tips (10-1000 μL), 1.5-mL microcentrifuge tubes, microcentrifuge tube rack, microcentrifuge
adapters, microcentrifuge, thermal cycler. Container with cracked or crushed ice, vortexer, 0.9%
saline solution (10 mL), 10% chelex 100 μL (in 0.2- or 0.5-mL PCR tube), micropipet and tips
(1-100 μL), thermal cycler, cheek cell, PV92B primer/loading dye mix 25 μL, Ready-To-Go
PCR beads (in 2.0-mL), mineral oil 5 mL, gel electrophoresis chamber, power supply, staining
trays, latex gloves, UV transilluminator, white light transilluminator, digital or instant camera,
water bath, pBR322, 1.5% agarose in 1×TBE 50 mL, 1×TBE 300 mL, ethidium bromide 250
mL, CarolinaBLU gel and buffer stain 7 mL, CarolinaBLU final stain 250 mL