LECTURE TEXT
First-order differential equations in chemistry
Gudrun Scholz •Fritz Scholz
Received: 7 August 2014 / Accepted: 13 September 2014 / Published online: 25 November 2014
ÓSpringer International Publishing 2014
Abstract Many processes and phenomena in chemistry,
and generally in sciences, can be described by first-order
differential equations. These equations are the most
important and most frequently used to describe natural
laws. Although the math is the same in all cases, the stu-
dent may not always easily realize the similarities because
the relevant equations appear in different topics and con-
tain different quantities and units. This text was written to
present a unified view on various examples; all of them can
be mathematically described by first-order differential
equations. The following examples are discussed: the
Bouguer–Lambert–Beer law in spectroscopy, time con-
stants of sensors, chemical reaction kinetics, radioactive
decay, relaxation in nuclear magnetic resonance, and the
RC constant of an electrode.
Keywords Differential equations Bouguer–Lambert–
Beer law Time constants Chemical kinetics
Radioactive decay Nuclear magnetic resonance RC
constant
Introduction
‘‘Differential equations are extremely important in
the history of mathematics and science, because the
laws of nature are generally expressed in terms of
differential equations. Differential equations are the
means by which scientists describe and understand
the world’’ [1].
The mathematical description of various processes in
chemistry and physics is possible by describing them with
the help of differential equations which are based on simple
model assumptions and defining the boundary conditions
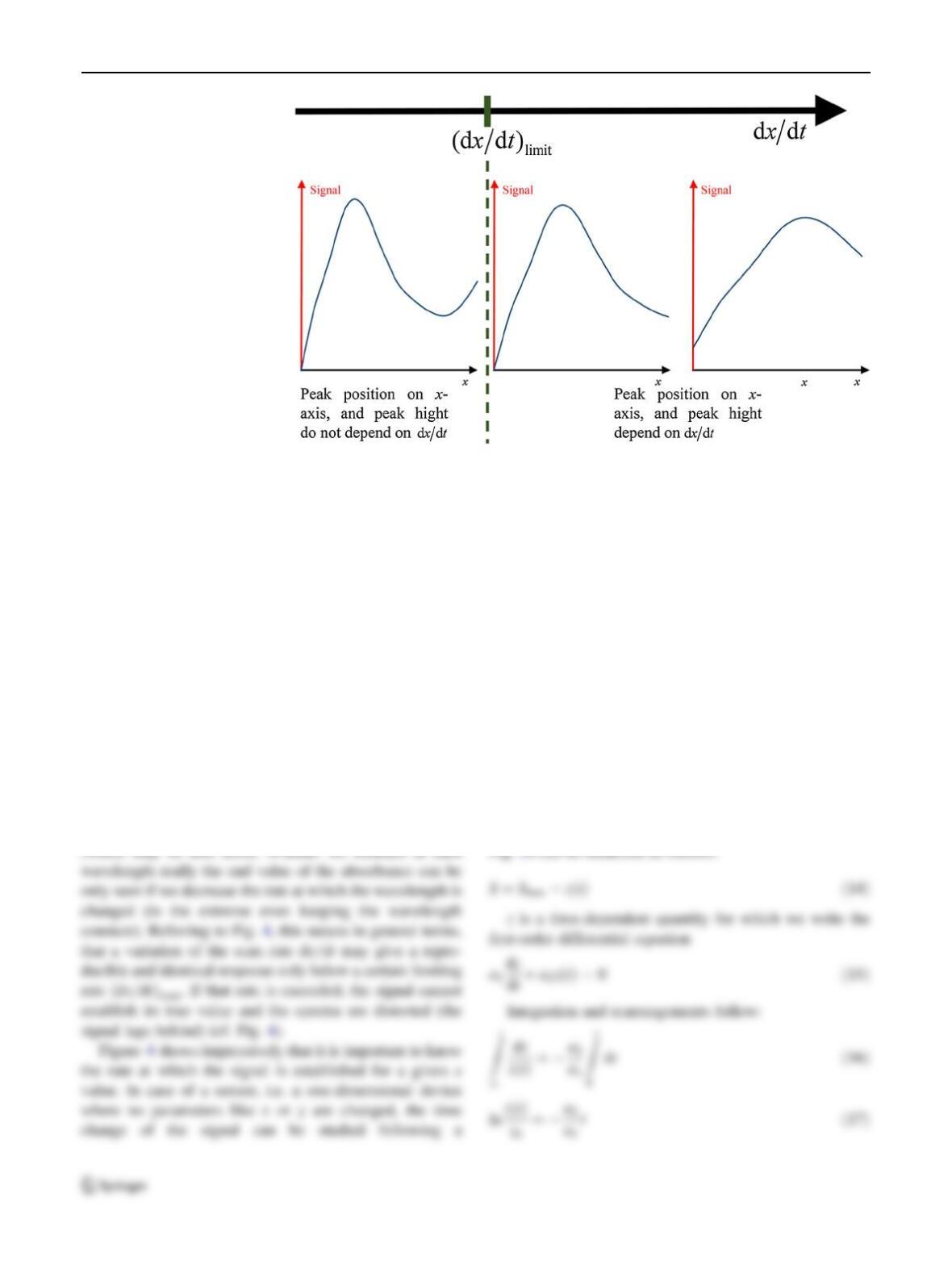
[2,3]. In many cases, first-order differential equations are
completely describing the variation dyof a function y(x)
and other quantities. If yis a quantity depending on x,a
model may be based on the following assumptions: The
differential decrease of the variable yis proportional to a
differential increase of the other variable, here x, i.e.
-dy*dx. This decrease -dyshould depend on the
function yitself: -dy*ydx, and together with a so far
unknown constant a, results in the equation
dy¼aydxð1Þ
Thus follows the ordinary linear homogeneous first-
order differential equation:
dy
dxþay ¼0ð2Þ
The characteristics of an ordinary linear homogeneous
first-order differential equation are: (i) there is only one
independent variable, i.e. here x, rendering it an ordinary
differential equation, (ii) the depending variable, i.e. here y,
having the exponent 1, rendering it a linear differential
equation, and (iii) there are only terms containing the
Electronic supplementary material The online version of this
article (doi:10.1007/s40828-014-0001-x) contains supplementary
material, which is available to authorized users.
G. Scholz
Department of Chemistry, Humboldt-Universita
¨t zu Berlin,
Brook-Taylor-Str. 2, 12489 Berlin, Germany
F. Scholz (&)
Institute of Biochemistry, University of Greifswald,
Felix-Hausdorff-Str. 4, 17487 Greifswald, Germany
e-mail: fscholz@uni-greifswald.de
123
ChemTexts (2014) 1:1
DOI 10.1007/s40828-014-0001-x