Question: Give the formula for stannous nitrate
A) Sn(NOQuestion:3
B) Sn(NOQuestion:3
C) Sn(NOQuestion:2
D) Sn(NOQuestion:2
E) Sn2(NOQuestion:
Answer:View Answer
Question: What is the approximate pH at the equivalence point of a weak
acid-strong base titration if 25 mL of aqueous formic acid
requires 29.80 mL of 0.3567 M NaOH? Ka=1.8 10-4 for formic acid.
A) 2.06
B) 5.48
C) 8.52
D) 11.94
Answer:View Answer
Question: Give all possible values of l for a 2 sublevel.
A) 2
B) -1/2
C) 0, 1
D) -1, 0, 1
E) 1, 2
Answer:View Answer
Question: How many moles of N2O4are in 76.3 g N2O4? The molar mass of
N2O4is 92.02 g/mol.
A) 7.02 x 103moles
B) 1.42 x 10-4moles
C) 1.00 mole
D) 1.21 moles
E) 0.829 moles
Answer:View Answer
Question: Calculate the pH of a solution that is 0.295 M in sodium
formate (NaHCOQuestion: and 0.205 M in formic acid (HCO2H). The Kaof
formic acid is 1.77 10-4.
A) 3.910
B) 3.587
C) 13.84
D) 10.10
E) 4.963
Answer:View Answer
Question: Give the name for HNO3.
A) nitric acid
B) nitrous acid
C) hydrogen nitrate
D) hydrogen nitrite
E) hydrogen nitride
Answer:View Answer
Question: An important buffer in the blood is a mixture of ________.
A) sodium chloride and hydrochloric acid
B) hydrochloric acid and sodium hydroxide
C) carbonic acid and bicarbonate ion
D) acetic acid and bicarbonate ion
E) acetic acid and carbonate ion
Answer:View Answer
Question: An acetylene molecule contains 2 atoms of carbon. The number
2 represents how many significant figures?
A) one
B) two
C) three
D) infinite
Answer:View Answer
Question: Identify a cation.
A) An atom that has lost an electron.
B) An atom that has gained an electron.
C) An atom that has lost a proton and a neutron.
D) An atom that has gained a neutron.
Answer:View Answer
Question: Choose the polydentate ligand from the substances below.
A) oxalate ion
B) EDTA
C) nitrite ion
D) hydroxide ion
E) carbon monoxide
Answer:View Answer
Question: Which of the following is TRUE concerning point “D” on the
figure?
A) The alloy is composed of the BCC structure.
B) The alloy is composed of the FCC structure.
C) The alloy is composed of both FCC and BCC phases, with a
larger portion of FCC present.
D) The alloy is composed of both FCC and BCC phases, with a
larger portion of BCC present.
E) None of the above can be determined from the information
given.
Answer:View Answer
Question: Which ion does not have a noble gas configuration in
its ground state?
A) Sc3+
B) Al3+
C) Ga3+
D) As3-
Answer:View Answer
Question: Identify the description of an atom.
A) neutrons and electrons in nucleus; protons in orbitals
B) neutrons in nucleus; protons and electrons in orbitals
C) protons and neutrons in nucleus; electrons in orbitals
D) protons and electrons in nucleus; neutrons in orbitals
E) electrons in nucleus; protons and neutrons in orbitals
Answer:View Answer
Question: What is the oxidation number of the sulfur atom in K2SO4?
A) -2
B) +2
C) +4
D) +6
Answer:View Answer
Question: Identify the compound with the standard free energy of
formation equal to zero.
A) KBr(s)
B) H2(g)
C) NO(g)
D) O3(g)
E) It is hard to determine.
Answer:View Answer
Question: Place the following elements in order of
increasing electronegativity.
Ba Se Li
A) Ba < Li < Se
B) Li < Se < Ba
C) Ba < Se < Li
D) Se < Ba < Li
E) Se < Li < Ba
Answer:View Answer
Question: Determine which of the following pairs of reactants will
result in a spontaneous reaction at 25C.
A) I-(aq) + Fe2+(aq)
B) Ca(s) + Mg2+(aq)
C) H2(g) + Sn2+(aq)
D) Ag(s) + Sn2+(aq)
E) All of the above pairs will react.
Answer:View Answer
Question: Calculate the pH of a buffer that is 0.145 M HC2H3O2and 0.202
M KC2H3O2. The Kafor HC2H3O2is 1.8 10-5.
A) 4.89
B) 9.01
C) 4.74
D) 5.05
E) 4.60
Answer:View Answer
Question: Which of the following metals will dissolve in HCl?
A) Ba
B) Na
C) Mn
D) Al
E) all of the above
Answer:View Answer
Question: Which of the following represents a chemical property of
hydrogen gas?
A) It is gaseous at room temperature.
B) It is less dense than air.
C) It reacts explosively with oxygen.
D) It is colorless.
E) It is tasteless.
Answer:View Answer
Question: Write the name for CoS.
A) cobaltous sulfate
B) cobaltous sulfide
C) cobaltic sulfide
D) cobaltic sulfate
E) cobalt sulfide
Answer:View Answer
Question: The molecular weight of urea ((NHQuestion:2CO), a compound used as a
nitrogen fertilizer, is ________ amu (rounded to one decimal
place).
A) 44.0
B) 43.0
C) 60.1
D) 8.0
E) 32.0
Answer:View Answer
Question: Give the expression for the solubility product constant for
Cr2(COQuestion:3.
A) [Cr3+]2[CO32-]3
B) 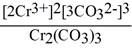
C) 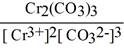
D) 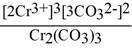
E) [3Cr3+]3[2CO32-]2
Answer:View Answer
Question: Identify the normal crystal structure at room temperature for
Cu.
A) face-centered cubic
B) trigonal planar
C) tetrahedral
D) hexagonal
E) hexagonal closest packed
Answer:View Answer
Question: Consider the following reaction:
Xe(g) + 2 F2(g) XeF4(g)
A reaction mixture initially contains 2.24 atm Xe and 4.27 atm
F2. If the equilibrium pressure of Xe is 0.34 atm, find the
equilibrium constant (Kp) for the reaction.
A) 25
B) 0.12
C) 0.99
D) 8.3
E) 0.040
Answer:View Answer
Question: A basketball is inflated to a pressure of 1.90 atm in a 24.0C
garage. What is the pressure of the basketball outside where the
temperature is -1.00C?
A) 1.74 atm
B) 1.80 atm
C) 2.00 atm
D) 2.08 atm
Answer:View Answer
Question: Which one of the following salts, when dissolved in water,
produces the solution with the highest pH?
A) KHCO3
B) CsClO4
C) RaO
D) CH3CH3NH3Cl
Answer:View Answer
Question: What decimal power does the abbreviation pico represent?
A) 1 106
B) 1 109
C) 1 10-1
D) 1 10-12
E) 1 10-15
Answer:View Answer
Question: What is the difference between an a-glycosidic linkage and a
b-glycosidic linkage? What implication does this have for the
strength of the intermolecular forces between molecules?
Answer:View Answer
Question: How will equilibrium be affected, with the same number of
moles of gas on both sides, if the pressure is increased?
Answer:View Answer
Question: Give the name of the instrument that is used to measure
masses of atoms and the percent abundance of isotopes.
Answer:View Answer
Question: What is a catalyst and what function does it serve?
Answer:View Answer
Question: Describe a molecule that can be a Lewis acid.
Answer:View Answer
Question: Determine the oxidation state of Mn in KMnO4.
Answer:View Answer
Question: Define random error.
Answer:View Answer