specify the capacity of the general ventilation system required to maintain a
steady state condition throughout this process area that protects against both
health and safety hazards due to ethylene glycol. For your information in
performing calculations the following data is provided:
ETHYLENE GLYCOL (CH2OHCH2OH)
Molecular weight: 62.1
Boiling point: 197.5o Celsius
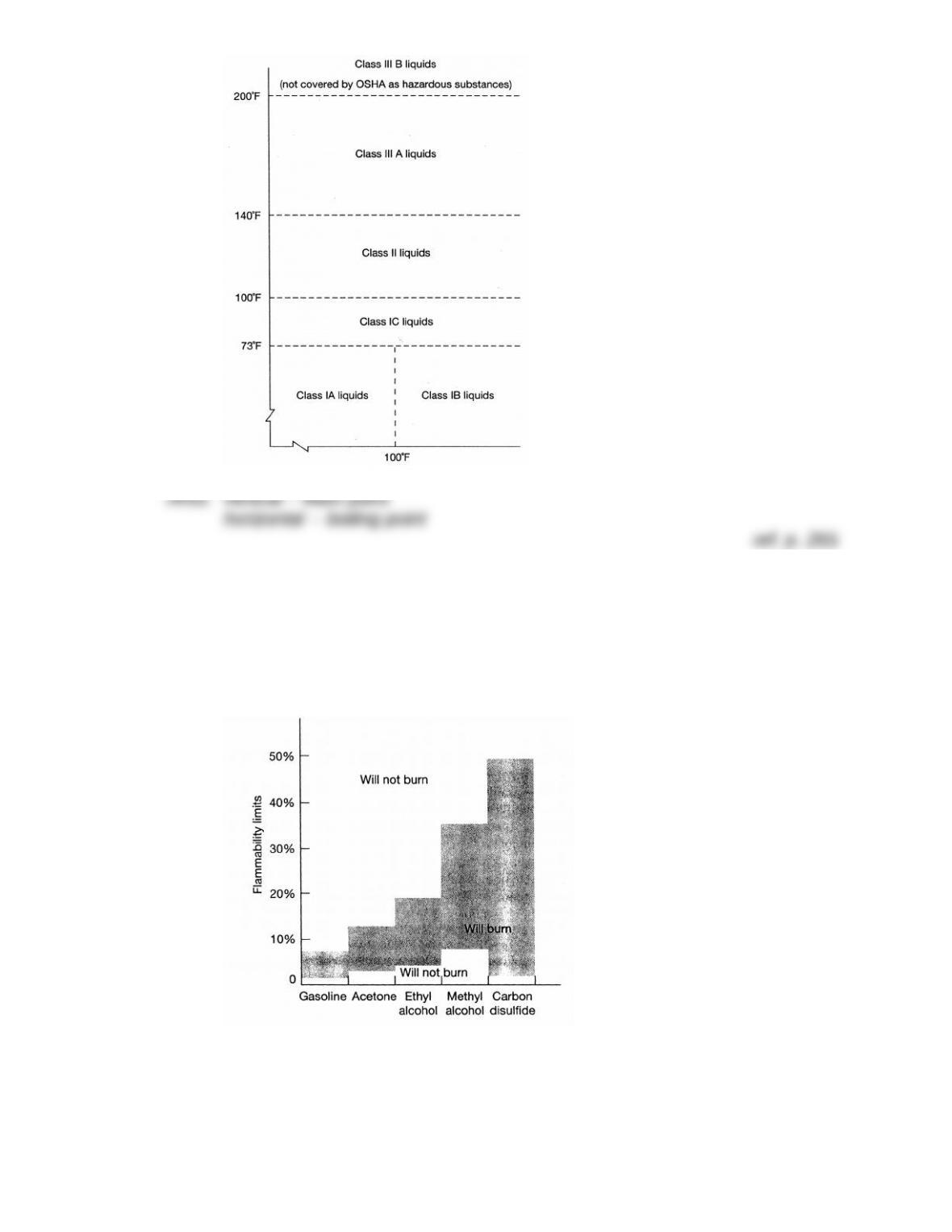
LEL: 3.2%
Firepoint: -13o Celsius
Flash point: 232o Fahrenheit
Autoignition temperature: 752o Fahrenheit
Vapor Pressure: 0.05 mm @ 20o Celsius
PEL: 50 ppm (Ceiling)
(a) Calculate how much exhaust ventilation (in cubic feet per hour,
general dilution type) is required to maintain safety hazards below
explosive levels. Show your work.
(b) Calculate how much exhaust ventilation (in cubic feet per hour,
general dilution type) is required to maintain health hazards below
OSHA-specified Action Levels (AL). Show your work.
2.4/vs = 3.2% = 0.032
vs = 2.4/0.032 = 160 ft3/hr
b) To maintain health hazards below acceptable levels, the PEL is the
design parameter. The PEL is shown above to be 50 ppm. Let vh represent the
required ventilation for health:
2.4/vh = 50 ppm = 0.000050
vh = 2.4/0.000050 = 48000 ft3/hr
(see continuation on next page)
(c) Calculate how many plant area room changes per hour the
level of ventilation calculated in part (b) would represent. Show your
work.