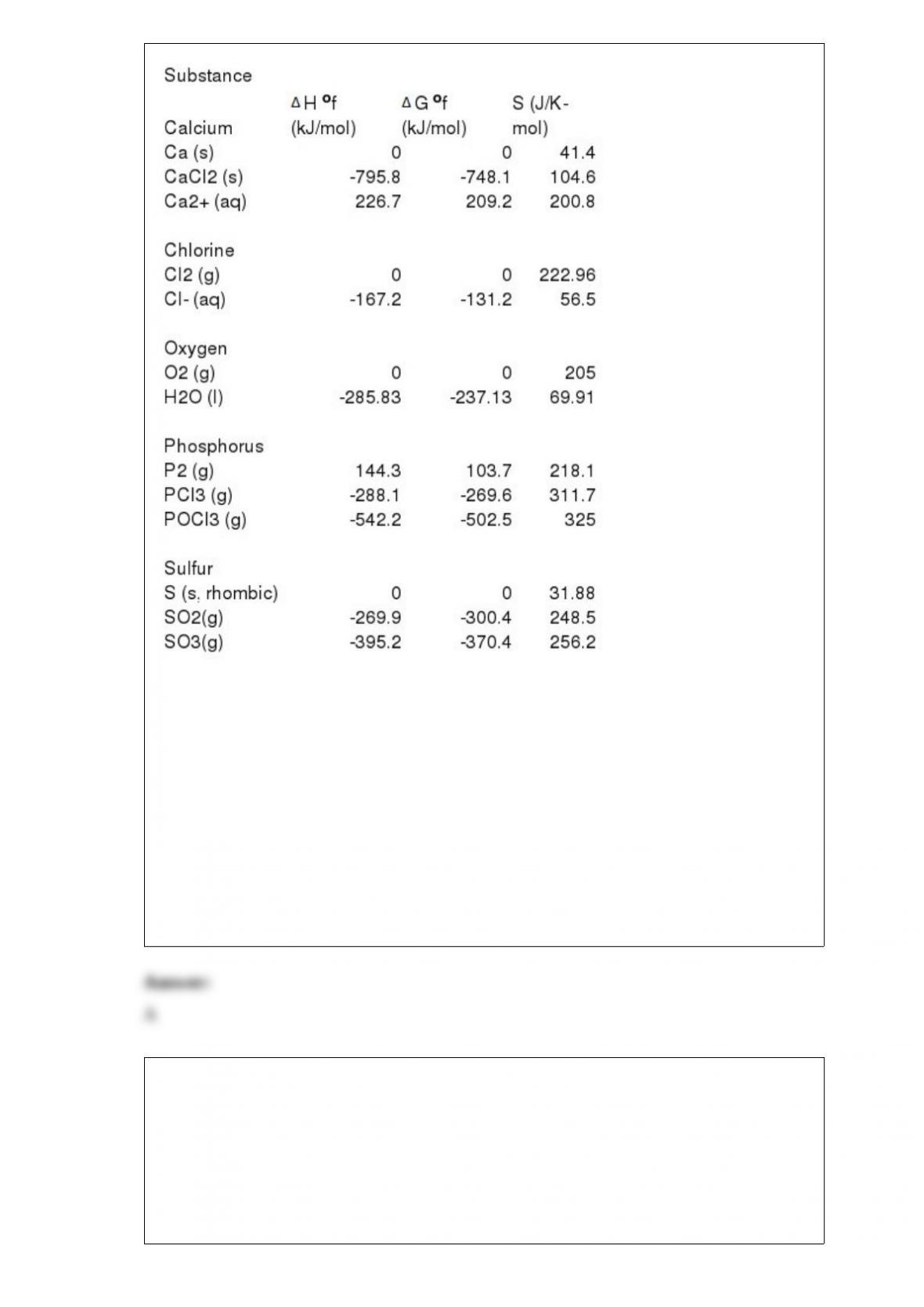
13) A sample of O2 gas (2.0 mmol) effused through a pinhole in 5.0 s. It will take
________ s for the same amount of CO2 to effuse under the same conditions.
A) 4.3
B) 0.23
C) 3.6
D) 5.9
E) 6.9
14) A solution is prepared by adding 40.00 g of lactose (milk sugar) to 110.0 g of water
at 55 oC The partial pressure of water above the solution is ________ torr. The vapor
pressure of pure water at 55 oC is 118.0 torr. The MW of lactose is 342.3 g/mol.
A) 2.216
B) 125.5
C) 225.9
D) 115.8
E) 86.5
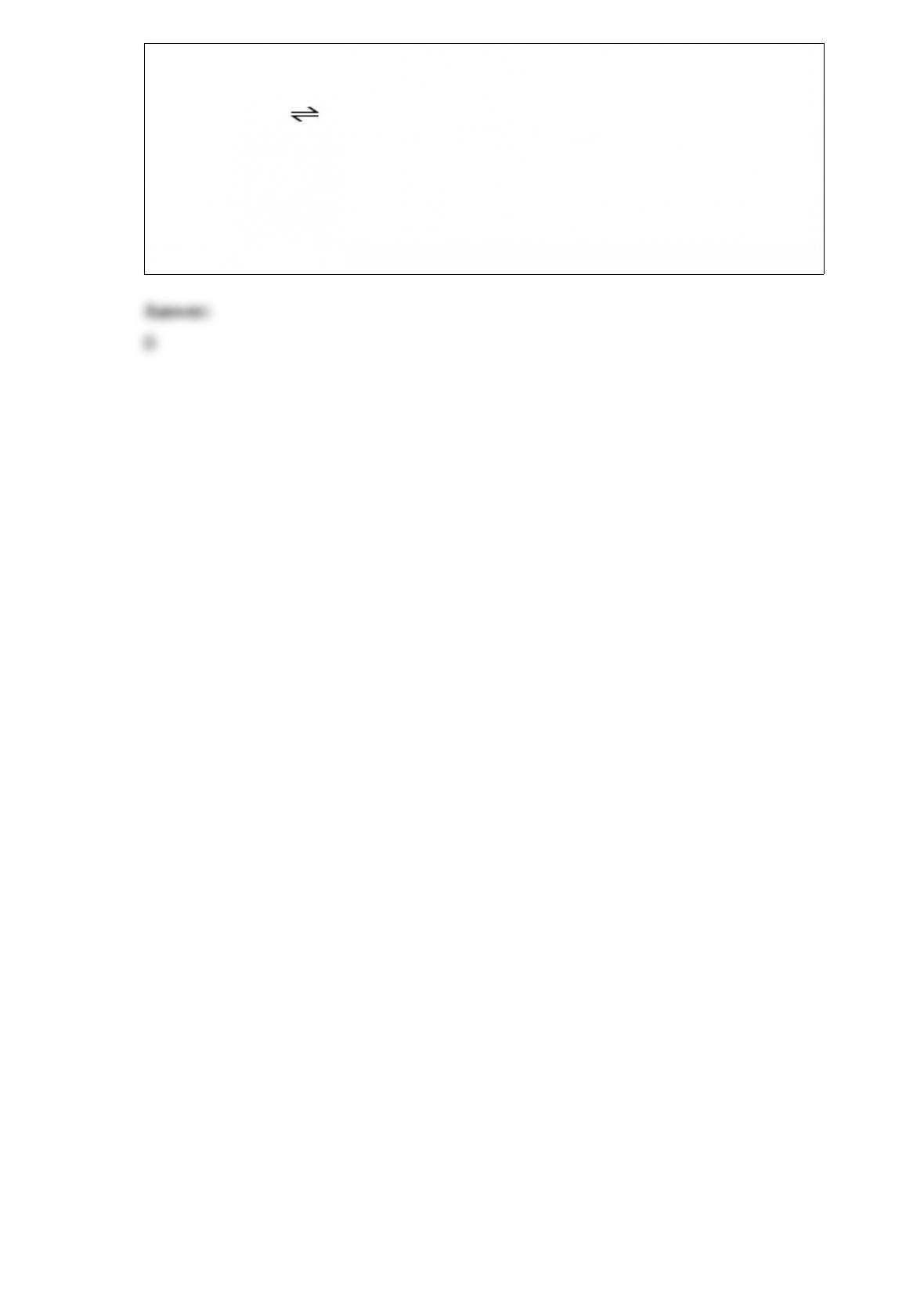
15) At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile
(CH3CN):
CH3NC (g) --> CH3CN (g)
At the start of the experiment, there are 0.200 mol of reactant (CH3NC) and 0 mol of
product (CH3CN) in the reaction vessel. After 25 min of reaction, 0.108 mol of reactant
(CH3NC) remain. The average rate of decomposition of methyl isonitrile, CH3NC, in
this 25 min period is ________ mol/min.
A) 3.7 x 10-3
B) 0.092
C) 2.3
D) 4.3 x 10-3
E) 0.54
16) The oxide of which of the following metals should have the greatest lattice energy?
A) calcium