1) What is the value of the reaction quotient, Q, for the voltaic cell
constructed from the following two half-reactions when the Zn2+
concentration is 0.0103 M and the Ag+ concentration is 1.35 M?
Zn2+(aq) + 2e Zn(s); e = 0.76 V
Ag+(aq) + e Ag(s); e = 0.80 V
A) 177
B) 131
C) ![]()
D) ![]()
E) ![]()
Answer: View Answer
2) How many moles of Fe(OH)2 [Ksp=1.81015] will dissolve in 1.0 liter
of water buffered at pH=10.37?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Answer: View Answer
3) The reduction potentials for Ni2+ and Sn2+ are as follows:
Ni2+ + 2e Ni, e = 0.231 V
Sn2+ + 2e Sn, e = 0.140 V
Calculate the equilibrium constant at 25 C for the reaction:
Sn2+ + Ni![]() Sn +
Sn +
Ni2+
A) ![]()
B) 35
C) 5.9
D) ![]()
E) ![]()
Answer: View Answer
4) Which of the following reactions is associated with the definition
of Kb?
A) Zn(OH2)62+![]() [Zn(OH2)5OH]+
[Zn(OH2)5OH]+
+ H+
B) CN + H+![]() HCN
HCN
C) F + H2O ![]() HF +
HF +
OH
D) Cr3+ + 6H2O ![]() Cr(OH2)63+
Cr(OH2)63+
E) none of these
Answer: View Answer
5) Which of the following is true in describing the crystal field
model?
A) The metal ion and ligand interaction is treated as a Lewis
acidbase interaction
B) The ligands are treated as negative point charges
C) The metal ionligand bonds are considered to be completely
ionic
D) The electrons are assumed to be localized
E) None of the above is true
Answer: View Answer
6) Which of the following is the second most abundant (by mass)
element in the earth’s crust, oceans, and atmosphere?
A) hydrogen
B) oxygen
C) carbon
D) aluminum
E) silicon
Answer: View Answer
7) What is the freezing point of an aqueous 1.58molal NaCl solution?
(Kf = 1.86C/m)
A) 2.94C
B) 2.94C
C) 5.88C
D) 5.88C
E) 0.00C
Answer: View Answer
8) What is the hybridization of the carbon atom that is double-bonded
to oxygen?
A) sp
B) sp2
C) sp3
D) dsp3
E) d2sp3
Answer: View Answer
9) Consider the titration of 300.0mL of 0.577M NH3 (Kb = 1.8105) with
500M HNO3. After 150.0mL of 0.500M HNO3 have been added, the pH of
the solution is:
A) 4.63
B) 11.37
C) 6.37
D) 9.37
E) none of these
Answer: View Answer
10) Which of the following statements is true concerning the
electrochemical cell described below at 25oC?
Cu | Cu2+(0.816 M) || Cu2+(0.843] M) | Cu
Cu2+(aq) + 2e Cu(s); e = 0.34 V
A) The cell reaction is spontaneous with a cell potential of 4.81
mV
B) The cell reaction is spontaneous with a cell potential of 0.418
mV
C) The cell reaction is nonspontaneous with a cell potential of 418
mV
D) The cell reaction is nonspontaneous with a cell potential of 81
mV
E) The cell reaction is spontaneous with a cell potential of 0.340
V
Answer: View Answer
11) You fill a balloon with 2.50 moles of gas at 22C at a pressure of
62 atm. What is the volume of the balloon?
A) 15.7 L
B) 98.0 L
C) 37.4 L
D) 2.79 L
E) 22.4 L
Answer: View Answer
12) Consider a 0.70 M solution of HOCl. If the molarity was decreased
to 0.3 M, which of the following statements would be true?
A) The percent dissociation would not change
B) The percent dissociation would increase
C) The percent dissociation would decrease
D) The equilibrium constant would stay the same
E) Two of these
Answer: View Answer
13) In which of the following compounds does N have its maximum
oxidation state?
A) N2O5
B) N2O
C) NO
D) N2O3
E) NO2
Answer: View Answer
14) Order the intermolecular forces (dipole-dipole, London dispersion,
ionic, and hydrogen-bonding) from weakest to strongest .
A) dipole-dipole, London dispersion, ionic, and
hydrogen-bonding
B) London dispersion, dipole-dipole, hydrogen-bonding, and
ionic
C) hydrogen-bonding, dipole-dipole, London dispersion, and
ionic
D) dipole-dipole, ionic, London dispersion, and
hydrogen-bonding
E) London dispersion, ionic, dipole-dipole, and hydrogen-bonding
Answer: View Answer
15) The following two reactions are important in the blast furnace
production of iron metal from iron ore (Fe2O3):![]()
Using these balanced reactions, how many moles of O2 are required
for the production of 3.19kg of Fe?
A) 42.8 moles
B) 19.0 moles
C) 171 moles
D) 57.1 moles
E) 2.39 moles
Answer: View Answer
16) What is the order of the reaction with respect to A?
A) 0
B) 1
C) 2
D) 3
E) 4
Answer: View Answer
17) Classify the following molecule: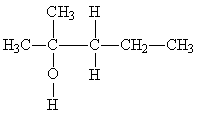
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) ether
E) phenol
Answer: View Answer
18) Name the following: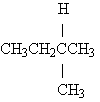
A) isopropane
B) methylpentane
C) methylbutane
D) n-pentane
E) dodecane
Answer: View Answer
19) Which types of processes are likely when the neutron-to-proton
ratio in a nucleus is too low?
| I. | a decay | ||
| II. | b decay | ||
| III. | positron production | ||
| IV. | electron capture | ||
A) I, II
B) II, III
C) III, IV
D) II, III, IV
E) II, IV
Answer: View Answer
20) What is the specific heat capacity of silver if it requires 86.3J
to raise the temperature of 15 grams of silver by 25C?
A) 4.3 J/gC
B) 0.23 J/gC
C) 0.14 J/gC
D) 0.60 J/gC
E) none of these
Answer: View Answer
21) For the process of a certain liquid vaporizing at 1 atm, DHvap =
2kJ/mol and DSvap=74.1J/mol K. Assuming these values are
independent of T, what is the normal boiling point of this
liquid?
A) 731 C
B) 1004 C
C) 458 C
D) 0.731 C
E) none of these
Answer: View Answer
22) Which statement is inconsistent with the kinetic theory of an ideal
gas?
A) The forces of repulsion between gas molecules are very weak or
negligible
B) Most of the volume occupied by a gas is empty space
C) When two gas molecules collide, they both gain kinetic
energy
D) The average kinetic energy of a gas is proportional to the
absolute temperature
E) Gas molecules move in a straight line between collisions
Answer: View Answer
23) Convert 34.4 lb to g. (1 lb = 453.6 g)
A) ![]() g
g
B) ![]() g
g
C) ![]() g
g
D) ![]() g
g
E) ![]() g
g
Answer: View Answer
24) A metal crystallizes in a body-centered unit cell with an edge
length of 2.00 102 pm. Assume the atoms in the cell touch along the
cube diagonal. The percentage of empty volume in the unit cell will
be:
A) 0%
B) 26.0%
C) 32.0%
D) 68.0%
E) none of these
Answer: View Answer
25) In the hydrogen spectrum, what is the wavelength of light
associated with the n = 3 to n=1electron transition?
A) 3.97 1025 nm
B) 8.21 102 nm
C) 9.75 106 cm
D) 1.94 1018 m
E) 1.03 107 m
Answer: View Answer
26) How many isomers are there of “dichloroethene”?
A) 2
B) 3
C) 4
D) 5
E) 6
Answer: View Answer
27) The phenomenon called __________ contraction is responsible for the
great similarity in atomic size and chemistry of 4d and 5d
elements.
A) transition
B) coordination
C) lanthanide
D) isomeric
E) none of these
Answer: View Answer
28) At 500.0K, one mole of gaseous ONCl is placed in a one-liter
container. At equilibrium it is 5.3% dissociated according to the
equation shown here: 2ONCl![]() 2NO+Cl2.
2NO+Cl2.
Determine the equilibrium constant.
A) 8.3 105
B) 1.6 103
C) 5.6 102
D) 9.5 101
E) 1.2 104
Answer: View Answer
29) A gas sample is held at constant pressure. The gas occupies 3.62 L
of volume when the temperature is 21.6C. Determine the temperature
at which the volume of the gas is 3.42 L.
A) 312 K
B) 278 K
C) 20.4 K
D) 295 K
E) 552 K
Answer: View Answer
30) Consider the reaction:
2ClF3(g) + 2NH3(g) N2(g) + 6HF(g) + Cl2(g)
When calculating the DHrxn, why is the DHf for N2 not
important?
A) Because nitrogen is in its standard elemental state and no
energy is needed for this product to exist
B) Because any element or compound in the gaseous state requires a
negligible amount of energy to exist
C) Because the products are not included when calculating DHrxn
D) Because nitrogen is in its elemental state and does not
contribute to the reaction itself
E) Two of the above statements explain why N2 is not important when
calculating DHrxn
Answer: View Answer
31) Name the following: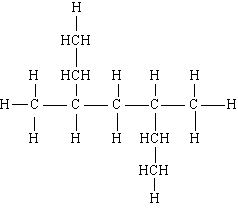
A) 2,4-diethylpentane
B) 3,5-dimethylheptane
C) secondary ethylpentane
D) 2,3-dimethyl-2,3-diethylpropane
E) none of these
Answer: View Answer
32) Which of the following statements is (are) false?
| I. |
The layering in a hexagonal closest-packed structure is aba. |
| II. |
A body-centered cubic unit cell has four atoms per unit cell. |
| III. |
For unit cells having the same edge length, a simple cubic structure would have a smaller density than a body-centered cube. |
| IV. |
Atoms in a solid consisting of only one element would have six nearest neighbors if the crystal structure were a simple cubic array. |
A) I
B) II
C) II, III
D) I, IV
E) II, III, IV
Answer: View Answer
33) What is the kinetic energy of a 1.56-kg object moving at
0km/hr?
A) 5.32 102 kJ
B) 6.89 103 kJ
C) 5.32 104 kJ
D) 1.06 103 kJ
E) 2.04 101 kJ
Answer: View Answer
34) This molecule is toxic because it has about 200 times the affinity
for the Fe2+ in hemoglobin as oxygen does, causing asphyxiation if
enough of it is present in the air.
A) CN
B) CO
C) CO2
D) NH3
E) CH4
Answer: View Answer
35) Calculate the mole fraction of NaCl in a solution prepared by
dissolving 117 g NaCl in 1.15kg H2O.
A) 9.90 101
B) 1.11 102
C) 6.08 102
D) 1.52 102
E) 3.04 102
Answer: View Answer
36) The process of transforming N2 to a form usable by animals and
plants is called
A) nitrogen fixation
B) fertilization
C) denitrification
D) the Ostwald process
E) nitrogenation
Answer: View Answer